digene HC2 High-Risk HPV DNA Test
For detection of human papillomavirus infections using Hybrid Capture 2 technology
For detection of human papillomavirus infections using Hybrid Capture 2 technology
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 5197-1330
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
The digene HC2 High-Risk HPV DNA Test is CE-IVD marked (see CE-IVD mark) for in vitro diagnostic use.
The digene HC2 High-Risk HPV DNA Test shows sensitivity of approximately 93% and a negative predictive value of nearly 95% in women who have had Pap test results of LSIL (low-grade squamous intrapethelial lesion), HSIL (high-grade squamous intraepithelial lesion), or equivalent (see table).
| Referral Pap LSIL or HSIL progression to HSIL disease | ||||
| + | – | Total | ||
| High-Risk HPV probe result | + | 89 | 140 | 229 |
| – | 7 | 91 | 98 | |
| Total | 96 | 231 | 327 | |
Using Hybrid Capture 2 technology for HPV testing offers the following benefits when compared with PCR for HPV testing:
The digene HC2 High-Risk HPV DNA Test detects the presence of 13 high-risk HPV types (16/18/31/33/35/39/45/51/52/56/58/59/68, which are carcinogenic) using full genome probes complementary to HPV DNA, specific antibodies, signal amplification, and chemiluminescent detection. It analyzes HPV DNA high-risk groups in cervical specimens.
The digene HC2 High-Risk HPV DNA Test has been approved for use with the digene HC2 DNA Collection Device, digene HC2 Sample Conversion Kit, digene Specimen Transport Medium, and PreservCyt Solution (Hologic). Automated options for the digene HC2 High-Risk HPV DNA Test include the Rapid Capture System (for high-volume sample throughput testing); guidelines vary among countries.
The digene HC2 High-Risk HPV DNA Test uses Hybrid Capture 2 technology to detect HPV. It is the most widely accepted HPV test, providing validation in conjunction with Pap in clinical studies.
The digene HC2 High-Risk HPV DNA Test detects the presence of 13 high-risk types using full genome RNA probes complementary to the HPV DNA, specific antibodies, and chemiluminescent detection. The target DNA combines with specific RNA probes, creating RNA:DNA hybrids. Then, the RNA:DNA hybrids are captured onto a solid phase coated with universal capture antibodies specific for RNA:DNA hybrids. The specimen matrix is then washed from the captured hybrids to remove inhibitors. During the signal amplification, captured RNA:DNA hybrids are detected with multiple antibodies conjugated to alkaline phosphatase. The signal resulting from the chemiluminescent reaction is read and the results are automatically interpreted.
The digene HC2 High-Risk HPV DNA Test should be used as an initial population screening test, with or without a Pap test, and to screen patients with ASC-US (atypical squamous cells of undetermined significance) results to determine the need for referral to colposcopy. The results of this test are not intended to prevent women from proceeding to colposcopy.
Guidelines for use of the digene HC2 High-Risk HPV Test vary among countries, but in many countries, experts recommend that women 30 years of age and older get the HPV Test along with the Pap test. This is the age group in which HPV infections are most likely to be persistent and result in cervical cancer. The presence or absence of high-risk HPV types, together with the physician's risk assessment of cytology history, other risk factors, and professional guidelines, may be used to guide patient management.
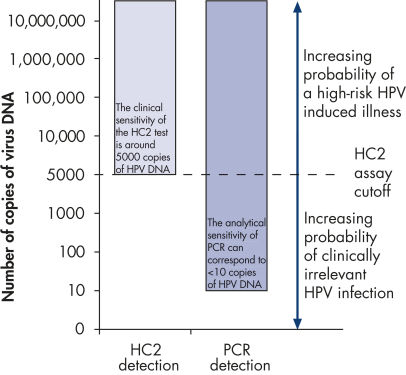
Clinical vs. analytical sensitivity. The clinically validated cutoff value of the HC2 test (5000 copies) has been established after extensive clinical research.