Products

artus BK Virus QS-RGQ Kit (24) CE
Cat. No. / ID: 4514363

artus BK Virus RG PCR Kit (24) CE
Cat. No. / ID: 4514263

artus BK Virus RG PCR Kit (96) CE
Cat. No. / ID: 4514265
Features
- Complete CE-IVD-compliant workflow (QIAsymphony RGQ)
- High reliability using the internal control
- Highly sensitive detection of as few as 26.7 copies/ml (QS-RGQ kit)
- Accurate quantitation using the 4 standards supplied
Product Details
Performance
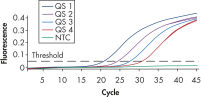
To ensure highest sensitivity, artus BK Virus Kits have been optimized to detect low numbers of BK virus DNA. The analytical sensitivity of the artus BK Virus QS-RGQ Kit is 26.7 copies/ml in consideration of the purification from plasma and assay setup using the QIAsymphony RGQ (see figure " Highly sensitive detection of BK virus DNA"). The specificity of the kits has been tested with the relevant BK virus strains.
| Kit | artus BK Virus RG PCR Kit | artus BK Virus QS-RGQ Kit |
|---|---|---|
| Validated sample types | Human plasma or urine | Human plasma or urine |
| Analytical sensitivity | 0.195 copies/μl in the PCR | 26.7 copies/ml in consideration of the purification (plasma) |
| Linear range | n.d. | 5.00 x 101 to 9.26 x 107 copies/ml (plasma) |
| Specificity | BK virus (tested with strains Dunlop, Gardner, AB269822, and S72390) | BK virus (tested with strains Dunlop, Gardner, AB269822, and S72390) |
See figures
Principle
The artus BK Virus RG PCR Kit and the artus BK Virus QS-RGQ Kit are based on the amplification and simultaneous detection of a specific region of the BK virus genome using real-time PCR. The kits provide high levels of specificity, sensitivity (see figure " Highly sensitive detection of BK virus DNA"), and reproducibility.
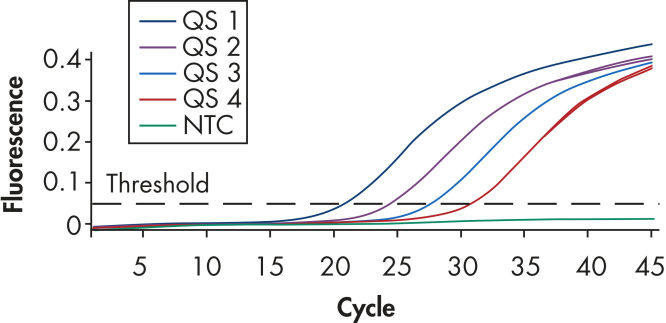
Each artus BK Virus Kit provides 4 BK virus quantitation standards (see figure "Reliable quantitation of BK virus load"). Use of the standards enables accurate quantitation of viral load. In addition, the kits contain a second heterologous amplification system to identify possible PCR inhibition. This is detected as an internal control (IC) in a different fluorescence channel from the analytical PCR. The detection limit of the analytical BK virus PCR is not reduced.
| Kit | artus BK Virus RG PCR Kit and artus BK Virus QS-RGQ Kit |
| Validated sample types | Human plasma or urine |
| Amplicon | 274 bp region covering parts of the VP2 and VP3 genes |
See figures
Procedure
artus BK Virus Kits provide all necessary reagents optimized for reliable BK virus DNA detection and quantitation. Simply add template DNA to the ready-to-use PCR master mix and Mg solution, and start the reaction on the appropriate real-time cycler using the optimized cycling program described in the kit handbook.
Common temperature profiles for multiple assays in one run
The temperature profile of artus BK Virus Kits corresponds to the profiles of the artus RG PCR Kits and artus QS-RGQ Kits for EBV, CMV, HSV-1/2, and VZV. Therefore, these PCR assays can be performed and analyzed in a single run.
Complete automated system from sample to BK virus detection
The QIAsymphony RGQ workflow solution for BK virus detection comprises the QIAsymphony SP for sample preparation, the QIAsymphony AS for assay setup, and the artus BK Virus QS-RGQ Kit on the Rotor-Gene Q. The system enables reliable pathogen detection with a complete CE-IVD-compliant workflow (see figure " Integrated QIAsymphony RGQ system for BK virus detection").
Recommendations for viral DNA purification when using the artus BK Virus RG PCR Kit
The artus BK Virus RG PCR Kit is validated for use with the EZ1 DSP Virus Kit. The artus BK Virus RG PCR Kit is also available as CE-IVD-marked EASYartus BK Virus RG PCR Kits, which include the EZ1 DSP Virus Kit for automated viral nucleic acid purification.
See figures
Applications
The artus BK Virus RG PCR Kit enables rapid and sensitive detection of BK virus DNA from human plasma or urine samples with highly accurate quantification. The kit is available for use on Rotor-Gene Q instruments.
The artus BK Virus QS-RGQ Kit is designed to be used with the QIAsymphony RGQ system, providing a complete CE-IVD-compliant workflow from plasma or urine samples to BK virus DNA detection and quantitation.
Supporting data and figures
Reliable quantitation of BK virus load.