RT2 PreAMP Pathway Primer Mixes
For amplification of cDNA templates prior to analysis on RT² Profiler PCR Arrays
For amplification of cDNA templates prior to analysis on RT² Profiler PCR Arrays
Cat. No. / ID: 330241
RT² PreAMP cDNA Synthesis Kit and RT² PreAMP Pathway Primer Mixes are a novel technology enabling gene expression analysis using as little as 1 ng total RNA. A proprietary amplification process increases the amount of cDNA for subsequent PCR array analysis. Starting sample types include fine needle biopsy and laser captured microdissection samples, stem cell clusters or embryoid bodies, and fluorescence-activated cell sorter (FACS) generated cell populations. Primer mixes are available for all cataloged human, mouse, and rat RT2 Profiler PCR Arrays.
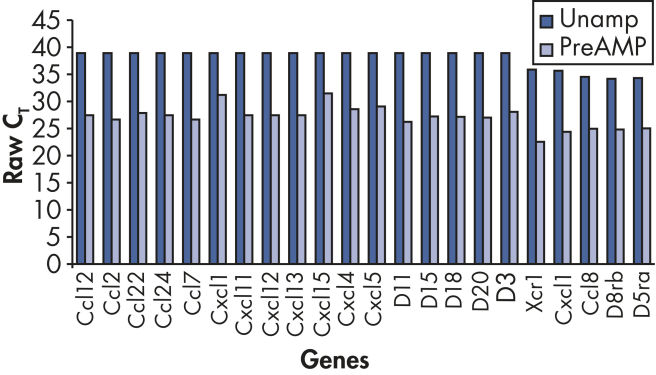
More genes are detected after preamplification using the RT² PreAMP Pathway Primer Mix (see figure " Increased positive call rate").
The amplification process is unbiased, resulting in highly comparable ΔCT values between preamplified and unamplified cDNA, as evaluated by regression analysis (see figure " Unbiased amplification process").
There is a high correlation of gene expression fold change results between preamplified and unamplified samples (see figure " Faithfully amplified biology").
First strand cDNA is first synthesized from up to 12 different RNA samples using the RT2 PreAMP cDNA Synthesis Kit. The cDNA is then preamplified for a pathway-specific set of genes. Each first strand cDNA synthesis reaction can be amplified using up to 4 different RT2 PreAMP Pathway Primer Mixes, allowing gene expression analysis on up to 4 different RT2 Profiler PCR Arrays. The Side Reaction Reducer included in the RT2 PreAMP cDNA Synthesis Kit eliminates the residual primers from preamplification, enabling accurate detection on RT2 Profiler PCR Arrays. To complete the PCR array procedure, preamplified templates are mixed with an instrument-specific, ready-to-use RT² SYBR® Green Mastermix.