✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
QIAamp MinElute Virus Spin Kit (50)
Cat. No. / ID: 57704
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Maximal concentration in eluate for high downstream sensitivity
- Rapid purification of high-quality viral DNA and RNA
- No organic extraction or alcohol precipitation
- Consistent, high yields
- Removal of contaminants and inhibitors
Product Details
QIAamp MinElute Virus Kits simplify viral DNA and RNA purification with fast spin-column and vacuum procedures. The kits use starting sample volumes of up to 0.2 ml and combine the selective binding properties of a silica-based membrane with flexible elution volumes of between 20 and 150 μl. The QIAamp MinElute Virus Spin process can be fully automated on the QIAcube Connect. The additional buffers and reagents in the QIAamp MinElute Virus Accessory are required for automation of the QIAamp MinElute Virus Spin procedure.
Performance
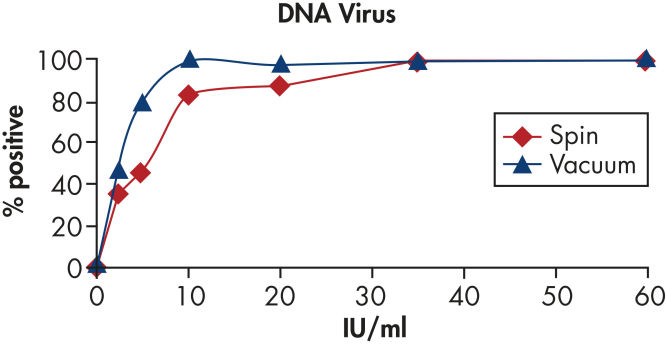
Purified viral nucleic acids are free of proteins, nucleases and other impurities, and are suitable for use in sensitive downstream applications such as PCR and RT-PCR (see figures " High sensitivity in PCR" and " High sensitivity in RT-PCR").
Viral nucleic acids purified using QIAamp MinElute Virus Kits can be used in a wide range of downstream applications, including:
- PCR and quantitative real-time PCR
- Infectious disease research
See figures
Principle
QIAamp MinElute Virus Kits simplify the isolation of viral RNA and DNA from plasma, serum and cell-free body fluids with a fast spin-column or vacuum procedure. No phenol–chloroform extraction is required. Nucleic acids bind specifically to the QIAamp MinElute silica-gel membrane while contaminants pass through. PCR inhibitors, such as divalent cations and proteins, are completely removed in two efficient wash steps, leaving pure nucleic acids to be eluted in either water or a buffer provided with the kit. QIAamp MinElute technology yields viral DNA and RNA from serum, plasma and cell-free body fluids that are ready to use in PCR and blotting procedures.
QIAamp MinElute Virus Kits combine the selective binding properties of a silica-based membrane with flexible elution volumes of between 20 and 150 µl. The QIAamp MinElute Virus Spin procedure can be fully automated on the QIAcube Connect. The additional buffers and reagents provided in the QIAamp MinElute Virus Accessory Set are required.
QIAamp sample preparation technology is fully licensed.
Procedure
Optimized buffers lyse samples, stabilize nucleic acids and enhance selective DNA adsorption to the QIAamp MinElute membrane (see flowcharts " QIAamp MinElute Virus Spin procedure" and “ QIAamp MinElute Virus Vacuum procedure”). Alcohol is added and lysates loaded onto the QIAamp MinElute spin column. Wash buffers are used to remove impurities and viral nucleic acids are eluted in Buffer AVE, ready for use in amplification reactions or storage at –20ºC. Purified nucleic acids are free of proteins, nucleases and other impurities.
See figures
Applications
The QIAamp MinElute Virus Spin Kit uses well-established technology for simultaneous purification of viral RNA and DNA from fresh or frozen plasma, serum, other cell-free body fluids.
The QIAamp MinElute Virus Vacuum Kit uses well-established technology for simultaneous purification of viral RNA and DNA. Fresh or frozen plasma, serum and other cell-free body fluids (500 µl) can be processed in less than an hour.
Comparison of QIAamp MinElute Virus Kits
| Features | QIAamp MinElute Virus Spin Kit | QIAamp MinElute Virus Vacuum Kit |
|---|---|---|
| Applications | PCR, real-time PCR | PCR, real-time PCR |
| Elution volume | 20–150 µl | 20–150 µl |
| Format | MinElute columns | MinElute columns |
| Main sample type | Serum, plasma | Serum, plasma |
| Processing | Manual (centrifugation) | Manual (vacuum) |
| Purification of total RNA, miRNA, poly A+ mRNA, DNA or protein | Viral DNA, viral RNA | Viral DNA, viral RNA |
| Sample amount | 200 µl | 500 µl |
| Technology | Silica technology | Silica technology |
| Time per run or per prep | <1 hour | <1 hour |
| Yield | Varies | Varies |
Supporting data and figures
High sensitivity in PCR.