QIAcuity One, 2plex Device
Cat. No. / ID: 911001
Eigenschaften
- Vollintegriertes System
- Skalierbares Format (Geräte mit 1, 4 und 8 Platten)
- Modernste Multiplexing-Funktionen (bis zu 5-Plex)
- Flexibler Probendurchsatz
- Umfassende Ergebnisse in ca. 2 Stunden
Angaben zum Produkt
Das QIAcuity Digital PCR System liefert präzise und multiplexe Quantifizierungsergebnisse für den Nachweis von Mutationen, Kopienzahlvariation (Copy Number Variation, CNV), Genexpressionsstudien, Gen-Editing-Analysen und vieles mehr. Dieses auf Nanoplatten basierende System integriert nahtlos einen dPCR-Standardworkflow mit Partitionierung, Thermocycling und Bildgebung in einer vollautomatischen Plattform mit minimalem Zeitaufwand für den Benutzer.
Das System wird zusammen mit QIAcuity Nanoplates und Zubehör verwendet.
In der virtuellen Demo können Sie mehr über QIAcuity erfahren.
Leistung
Das QIAcuity Digital PCR System macht die absolute Quantifizierung für alle Labore zugänglich und erschwinglich. Die Vollautomatik integriert und optimiert den gesamten digitalen PCR-Workflow von Partitionierung, Thermocycling und Bildgebung in einem einzigen Gerät und das bei minimalem Zeitaufwand für den Benutzer. Darüber hinaus lassen sich Ihre aktuellen qPCR-Assays leicht an das QIAcuity Digital PCR System anpassen. Beim Umstieg von der qPCR ist keine Änderung der Plattenhandhabung erforderlich, was eine schnelle Assay-Konfiguration und rasche Ergebnisse in ca. 2 Stunden gewährleistet.
QIAcuity Geräte – Merkmale und Spezifikationen
| Merkmal | QIAcuity One | QIAcuity Four | QIAcuity Eight |
|---|---|---|---|
| Anzahl verarbeiteter Platten | 1 | 4 | 8 |
| Detektionskanäle (Multiplexing) | 2 oder 5 | 5 | 5 |
| Thermocycler | 1 | 1 | 2 |
| Zeit bis zum Ergebnis | Ca. 2 Std. |
Erste Platte ca. 2 Std. Jede folgende Platte alle ~60 Min. |
Erste Platte ca. 2 Std. Jede folgende Platte alle ~30 Min. |
| Durchsatz (verarbeitete Proben an einem Arbeitstag) |
Bis zu 384 (96-Well) Bis zu 96 (24-Well) |
Bis zu 672 (96-Well) Bis zu 168 (24-Well) |
Bis zu 1248 (96-Well) Bis zu 312 (24-Well) |
Prinzip
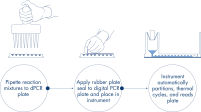
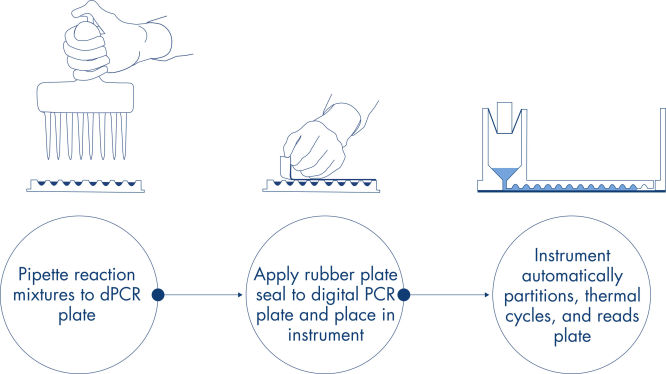
In nur 3 einfachen Schritten erhalten Sie in ca. 2 Stunden das gewünschte dPCR-Ergebnis: pipettieren und laden, Experiment durchführen, Ergebnisse auswerten.
Das Prinzip der dPCR-Reaktion in den Nanoplatten finden Sie hier beschrieben.
Verfahren
Genau wie bei qPCR-Experimenten umfasst die Probenvorbereitung den Transfer von Master-Mix, Sonden und Primern in eine 96- oder 24-Well-Nanoplatte, gefolgt von der Zugabe der Proben. Das System integriert Partitionierung, Thermocycling und Bildgebung in nur einem Vollautomaten, der den Benutzer in weniger als 2 Stunden von der Probe zum Ergebnis führt. Mit der Software Suite lassen sich Auswertungen durchführen, die die Konzentration der Zielsequenz in Kopien pro Mikroliter sowie Qualitätskontrollen wie positive Proben und NTC liefern. Diese Auswertung kann auch auf Remote-Computer innerhalb desselben lokalen Netzwerks (LAN) ausgedehnt werden.
Anwendungen
Zusammen mit den QIAcuity Nanoplates und QIAcuity PCR Kits ermöglichen die QIAcuity Geräte digitale PCR-Anwendungen, darunter:
- Nachweis seltener Mutationen
- Auswertung der Variation der Kopienzahl
- Genexpressionsanalyse
- Pathogennachweis
- Genotypisierung
- miRNA-Forschung
Software
Die mit dem Gerät gelieferte und auf einem separaten Computer installierte QIAcuity Software Suite steuert ein oder mehrere QIAcuity Geräte, die entweder direkt mit einem Gerät verbunden sind oder ein vorhandenes lokales Netzwerk (LAN) nutzen. Mit der QIAcuity Software Suite können digitale PCR-Experimente, Proben und Reaktionsmischungen definiert, Nanoplatten zugeordnet und auf das QIAcuity Gerät übertragen werden. Nach dem Gerätelauf können die Daten analysiert, Berichte erstellt und die Daten zur externen Auswertung exportiert werden. Die Software bietet multiple Template-Funktionen, um sich wiederholende Plattenlayouts und Plattenlaufparameter leicht aufrufen zu können und so Ihre Erfahrung mit der digitalen PCR weiter zu optimieren.
Bei Integration in ein lokales Netzwerk dient der Computer, auf dem die QIAcuity Software Suite installiert ist, als Server, auf den andere Computer als Clients über das LAN zugreifen können. So können mehrere Benutzer von anderen Räumen oder Büros aus auf die Software zugreifen und Daten über einen Standardbrowser analysieren, ohne dass die Software auf mehreren Computern installiert werden muss oder der Zugriff und Austausch von Daten über Internetverbindungen benötigt wird.
Services
Schützen Sie Ihr Gerät mit den vielfältigen Servicelösungen von QIAGEN. Erfahren Sie mehr über spezielle Serviceverträge, die auf Ihre Bedürfnisse abgestimmt sind.
Ergänzende Daten und Abbildungen
Ein einfacher und schneller plattenbasierter Workflow.
Service-Pläne
QIAcuity One 2plex Full Agreement
Cat. No. / ID: 9245362
QIAcuity One Preventive Subscription
Cat. No. / ID: 9245355
QIAcuity Four Preventive Subscription
Cat. No. / ID: 9245356
QIAcuity Eight Preventive Subscription
Cat. No. / ID: 9245357
QIAcuity One 2plex Core Agreement
Cat. No. / ID: 9245358
QIAcuity One 5plex Core Agreement
Cat. No. / ID: 9245359
QIAcuity Four Core Agreement
Cat. No. / ID: 9245360
QIAcuity Eight Core Agreement
Cat. No. / ID: 9245361
QIAcuity One 2plex Basic Agreement
Cat. No. / ID: 9245401
QIAcuity One 5plex Basic Agreement
Cat. No. / ID: 9245402
QIAcuity One 5plex Full Agreement
Cat. No. / ID: 9245363
QIAcuity One Installation & Training
Cat. No. / ID: 9245352
QIAcuity Four Installation & Training
Cat. No. / ID: 9245353
QIAcuity Eight Installation & Training
Cat. No. / ID: 9245354