✓ Automatische Verarbeitung von Online-Bestellungen 24/7
✓ Sachkundiger und professioneller technischer und Produkt-Support
✓ Schnelle und zuverlässige (Nach-)Bestellung
QIAcuity EG PCR Kit (1 ml)
Cat. No. / ID: 250111
✓ Automatische Verarbeitung von Online-Bestellungen 24/7
✓ Sachkundiger und professioneller technischer und Produkt-Support
✓ Schnelle und zuverlässige (Nach-)Bestellung
Eigenschaften
- Für digitale PCR-Reaktionen auf Farbstoffbasis mit EvaGreen
- 3x konzentrierter Master-Mix zum Laden von mehr Proben
- Optimiert für den mikrofluidischen Einsatz mit QIAcuity Nanoplates
- REACH-Konformität
Angaben zum Produkt
Das QIAcuity EG PCR Kit enthält einen 3x konzentrierten, gebrauchsfertigen Master-Mix, der für den mikrofluidischen Einsatz mit den QIAcuity Nanoplates optimiert ist. Das Kit steigert die Spezifität und Effizienz der farbstoffbasierten digitalen PCR und ermöglicht eine genaue Quantifizierungsanalyse. Der interkalierende Farbstoff EvaGreen bindet an Doppelstrang-DNA und erhöht die quantitative Genauigkeit der gDNA- und cDNA-Messungen auf den QIAcuity dPCR-Geräten.
Das Kit wird zusammen mit dem QIAcuity Digital PCR System und den QIAcuity Nanoplates eingesetzt.
Möchten Sie Näheres über das Produkt erfahren und von einem unserer dPCR-Spezialisten kontaktiert werden? Registrieren Sie sich hier, und wir werden uns umgehend mit Ihnen in Verbindung setzen.
Leistung
Überlegene Leistung
Die QIAcuity Master-Mixe für den EvaGreen-basierten Nachweis nutzen die neuesten Versionen der hochwertigen DNA-Polymerase von QIAGEN. Die einzigartige Kombination von QIAGENs proprietärer und bewährter Puffertechnologie, die für die Nanoplatten-Mikrofluidik optimiert wurde, mit der neuen QuantiNova DNA-Polymerase liefert äußerst beständige Ergebnisse hinsichtlich der Sensitivität, Reproduzierbarkeit und Effizienz.
Farbstoffbasierter Nachweis mit EvaGreen
Der spezielle Master-Mix im QIAcuity EG PCR Kit erlaubt die präzise Amplifikation und Quantifizierung doppelsträngiger DNA-Ziele. Hierzu gehört ein optimierter Referenzfarbstoff, der für die dPCR-Analyse und die Zählung auswertbarer Partitionen auf den Nanoplatten benötigt wird. EvaGreen liefert darüber hinaus bei gleichen Konzentrationen ein stärkeres Fluoreszenzsignal als SYBR Green und bietet maximale Amplifikationseffizienz, Spezifität und Sensitivität bei der dPCR.
Reaktionsstabilität von bis zu 100 Stunden
Die QIAcuity PCR-Mixe können bis zu 100 Stunden bei 30 °C gelagert werden, ohne dass dies die Leistung der nachfolgenden Reaktionen beeinträchtigt. Die auch nach längerer Lagerung ohne Kühlmittel bei Raumtemperatur bestehende hervorragende Stabilität ermöglicht den Einsatz des QIAcuity EG PCR Kit für die Arbeit mit Hochdurchsatzreaktionen und Plattenstapeln.
Prinzip
Durch seinen neuartigen, Antikörper-vermittelten Hot-Start-Mechanismus bietet das QIAcuity EG PCR Kit cDNA- und gDNA-Analysen höchster Spezifität. Bei niedrigen Temperaturen wird die QuantiNova-DNA-Polymerase durch den QuantiNova-Antikörper und QuantiNova Guard, ein neuartiges, den Komplex stabilisierendes Additiv, in einem inaktiven Zustand gehalten. Dies verbessert die Stringenz des Hot-Starts und verhindert die Verlängerung von unspezifisch gebundenen Primern und Primer–Dimeren. Innerhalb von 2 Minuten nach Temperaturerhöhung auf 95 °C werden der QuantiNova-Antikörper und QuantiNova Guard denaturiert und die QuantiNova-DNA-Polymerase wird aktiviert, was die PCR-Amplifikation ermöglicht.
Das Prinzip der dPCR-Reaktion in den Nanoplatten finden Sie hier beschrieben.
Verfahren
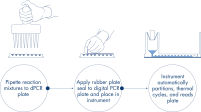
Genau wie bei qPCR-Experimenten umfasst die Probenvorbereitung den Transfer von Master-Mix, Sonden und Primern in eine 96- oder 24-Well-Nanoplatte, gefolgt von der Zugabe der Proben. Das System integriert Partitionierung, Thermocycling und Bildgebung in nur einem Vollautomaten, der den Benutzer in weniger als 2 Stunden von der Probe zum Ergebnis führt. Mit der Software Suite lassen sich Auswertungen durchführen, die die Konzentration der Zielsequenz in Kopien pro Mikroliter sowie Qualitätskontrollen wie positive Proben und NTC liefern. Diese Auswertung kann auch auf Remote-Computer innerhalb desselben lokalen Netzwerks (LAN) ausgedehnt werden.
Anwendungen
Im Zusammenspiel mit dem QIAcuity Digital PCR System und den QIAcuity Nanoplates ermöglicht das QIAcuity EG PCR Kit die quantitative Analyse von cDNA-Zielen und gDNA für den Einsatz in Anwendungen wie:
- Nachweis seltener Mutationen
- Auswertung der Variation der Kopienzahl
- Genexpressionsanalyse
- Pathogennachweis
- Genotypisierung
- miRNA-Forschung
Ergänzende Daten und Abbildungen
Ein einfacher und schneller plattenbasierter Workflow.