QuantiTect Primer Assay (200)
Cat. No. / ID: 249900
Features
- Guaranteed results with genomewide, predesigned primers
- ~100% PCR efficiencies for reliable relative quantification
- High sensitivity and specificity
- Accurate quantification over a wide linear range
- Time and cost savings with SYBR Green detection
Product Details
QuantiTect Primer Assays are genomewide, bioinformatically validated primer sets for use in SYBR Green-based real-time RT-PCR on any cycler. Assays are available for all genes from human, rat, mouse, and many other species. Each assay for a specific gene is supplied as a lyophilized mix of forward and reverse primers that can be easily reconstituted to obtain a 10x assay solution (reaction components for real-time RT-PCR need to be ordered separately). When used in combination with QuantiFast, QuantiTect, Rotor-Gene, or FastLane Kits for SYBR Green detection, QuantiTect Primer Assays guarantee highly specific and sensitive results in real-time RT-PCR that are comparable to probe-based detection.
Performance
QuantiTect Primer Assays enable reproducible quantification of RNA transcripts over a range of template amounts (see part [A] of figure " Reproducible real-time RT-PCR"). Accurate quantification is guaranteed when the assays are run using QuantiFast SYBR Green Kits or other SYBR Green kits from QIAGEN. The kits prevent the formation of PCR artifacts (see part [B] of figure " Reproducible real-time RT-PCR").
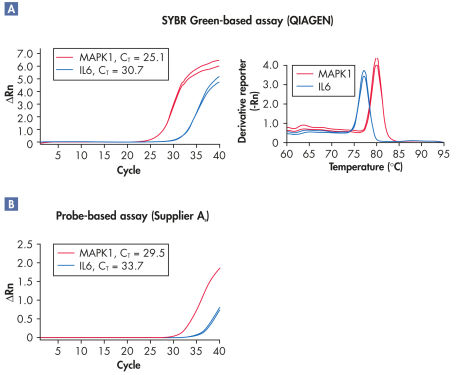
When used with QuantiFast, QuantiTect, Rotor-Gene, or FastLane SYBR Green Kits (master mixes for two-step or one-step RT-PCR), the assays may even give lower CT values compared with probe-based assays (see figure " Superior sensitivity in real-time RT-PCR"). This is due to the high PCR specificity of the kits and multiple binding of SYBR Green dye to each PCR product. Highly sensitive quantification is possible even with low-abundance transcripts.
See figures
Principle
See figures
Procedure
Each QuantiTect Primer Assay is supplied as a lyophilized mix of forward and reverse primers for a specific gene. The primers are reconstituted in TE, pH 8.0 to give a 10x primer solution, which is then added to the master mix supplied with a QuantiFast, QuantiTect, Rotor-Gene, or FastLane Kit for SYBR Green detection. The master mix with primers is aliquoted into PCR tubes or wells, and the individual template samples are then added.
QuantiTect Primer Assays provide guaranteed results in gene expression analysis when used in combination with one of the following kits shown in the table.
| Two-step qRT-PCR | One-step qRT-PCR |
|---|---|
| QuantiFast SYBR Green PCR Kit | QuantiFast SYBR Green RT-PCR Kit |
| Rotor-Gene SYBR Green PCR Kit | Rotor-Gene SYBR Green RT-PCR Kit |
| QuantiTect SYBR Green PCR Kit | QuantiTect SYBR Green RT-PCR Kit |
QuantiTect Primer Assays are designed to detect RNA only where possible. For assays where detection of genomic DNA cannot be avoided, cDNA can be prepared using the QuantiTect Reverse Transcription Kit or the FastLane Cell cDNA Kit. Both kits provide cDNA synthesis with integrated removal of genomic DNA contamination.
Naming
Each QuantiTect Primer Assay available for a particular gene is given a number that appears in the product name after the gene symbol. Read more in the GeneGlobe FAQs.
Applications
QuantiTect Primer Assays are for gene expression analysis using one-step and two-step qRT-PCR with SYBR Green detection. They are highly suited for applications such as:
- Validation of siRNA-mediated gene knockdown
- Validation of microarray results
- High-throughput Screening
Supporting data and figures
Superior sensitivity in real-time RT-PCR.
Specifications
| Features | Specifications |
|---|---|
| Applications | Gene expression analysis |
| Kit for this application on the instrument | QuantiFast SYBR Green PCR or RT-PCR Kit, QuantiTect SYBR Green PCR or RT-PCR Kit |
| Detection | SYBR Green I |
| Real-time or endpoint | Real-time |
| Species | Human, mouse, rat, drosophila arabidopsis, chicken, dog, nematode, and zebrafish |
| Format | Lyophylized primer set |