Products
For up-to-date licensing information or Rotor-Gene Q instrument specific disclaimers, refer to the corresponding Rotor-Gene Q instrument manual.
Features
- Standardized, fully automated real-time PCR analysis
- Maximized process safety and ease of use
- Unprecedented flexibility for all routine testing demands
- CE-IVD compliant QIAGEN assays (in preparation) and user-defined tests
- Enhanced lab productivity with optimized workflows
Product Details
Rotor-Gene AssayManager software is used in combination with Rotor-Gene Q MDx and QIAsymphony RGQ instruments for routine testing. This intuitive software manages all tasks associated with running real-time PCR assays offering enhanced convenience, standardization, and flexibility. Fully automated analysis and interpretation of results minimizes human error, delivering results you can trust. With an extensive range of artus QS-RGQ Kits for a CE-IVD compliant workflow (in preparation) as well as the flexibility of user defined tests, Rotor-Gene AssayManager covers the demands of routine testing laboratories.
Principle
Rotor-Gene AssayManager is able to read in sample information, set up experiments, control up to 4 different Rotor-Gene Q cyclers, acquire data from these instruments, automatically analyze results, and create reports. All information on how to run, analyze, interpret, and report an assay is stored in the easy-access Rotor-Gene AssayManager database. Comprehensive user guidance and well-defined workflows facilitate increased standardization and laboratory productivity. In addition, Rotor-Gene AssayManager supports the latest quality and usability requirements for software, such as 21 CFR Part 11.
Rotor-Gene AssayManager enables:
Rotor-Gene AssayManager enables:
- Operation of up to 4 Rotor-Gene Q cyclers from one computer
- Individual or network installation with remote access
- IVD-compliant data management
- IVD-compliant user management
- Data import/export from QIAsymphony instruments, LIMS, QIAGEN LIMS middleware
- Fully automated analysis and interpretation of results
- Validated CE-IVD workflows for artus infectious disease assays (in preparation)
- User-validated workflows (User Defined Test mode)
Procedure
Comprehensive user guidance with flexible options
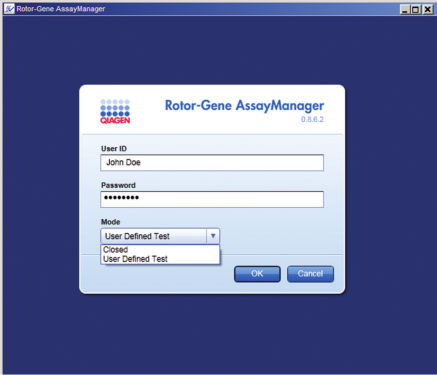
Rotor-Gene AssayManager offers 2 modes of operation to the user: the Closed Mode and the User Defined Test Mode. The user simply logs in to the software and selects whether to run an assay validated by QIAGEN in Closed Mode or an assay validated by the user in User Defined Test Mode (see figure " Easy login and assay selection"). Color schemes, easy-to-understand icons, and comprehensive help and warning messages guide the user step-by-step through the software.Control of up to 4 Rotor-Gene Q cyclers and remote access
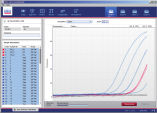
Rotor-Gene AssayManager controls the Rotor-Gene Q cycler, i.e., the software will provide all functions to set up, start, and run real-time PCR experiments on up to 4 Rotor-Gene Q cyclers in parallel (see figure " Increased lab efficiency"). This saves laboratory space and increases lab efficiency.Rotor-Gene AssayManager can also be used for experiment setup, result approval, or reporting on computers not connected to a Rotor-Gene Q cycler. In this case, the software can be installed on the user's office computer enabling convenient result review and approval. Alternatively, a computer in the assay setup room may be used for run setup. This networking capability together with secure database management simplifies a variety of everyday tasks.
The seamless connection of Rotor-Gene AssayManager to automated upstream sample processing and assay setup with QIAsymphony SP/AS instruments guarantees highest workflow convenience and process safety. In addition, manual up-front processing steps are supported by a special editor, step-by-step procedures, automatic barcode reading, and other features.
Fully automated real-time PCR analysis
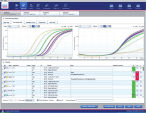
Automated real-time PCR analysis is the core function of Rotor-Gene AssayManager. Rotor-Gene AssayManager analyzes the real-time PCR raw data according to well-defined assay specific rules and generates result reports comprising information on the validity or invalidity of the assay and individual samples (see figure " Automated result reporting").For QIAGEN validated assays, the unique Automatic Data Scan (AUDAS) compares real-time data with performance characteristics established by statistical methods during assay development. This new feature detects assay deviations and artifacts that cannot be monitored by the assay controls alone, ensuring best-in-class, error-free real-time PCR analysis.
Reliable data management
Rotor-Gene AssayManager imports sample-specific information from QIAsymphony instruments, from a LIMS, from a dedicated QIAGEN LIMS middleware solution (in preparation), or from separate Rotor-Gene AssayManager installations. All data from all installations and from all Rotor-Gene Q cyclers in your lab can be stored in a central database. After analysis, approval, and reporting (e.g., as a printout, PDF, or LIMS export), the data are moved to a common electronic archive. For fast and easy data access of all data at each time point, the database is searchable by using various filter functions.Secure user management
Different user profiles are available and are password protected (user login is required) for increased security. Operators set up and start a run, while administrators are responsible for managing all assets (e.g., cyclers, assay profiles, users) necessary for working with Rotor-Gene AssayManager. Approvers accept or reject the results of a run and release the data of an experiment. Assignment of these dedicated user roles enables optimal use of resources for your laboratory tasks.Support of 21 CFR Part 11 compliance
Rotor-Gene AssayManager includes specific features that support the technical requirements of 21 CFR Part 11 regulations, enabling use of an electronic records system. User accounts are password-protected, including special security requirements, and all user actions are documented in an audit trail that can be filtered and printed out. The Rotor-Gene AssayManager audit trail is designed based on guidelines in FDA CFR Title 21, Part 11 Electronic Records, Electronic Signatures.Software that meets the demands of routine testing applications — today and tomorrow
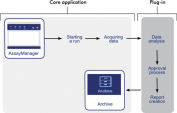
Rotor-Gene AssayManager standardizes procedures such as cycler control and data storage, helping to reduce the burden of multiple standard operating procedures and simplifying laboratory documentation (see figure " Standardized workflow"). Full flexibility is retained through the use of modular plug-ins that ensure future assays and different assay designs can be run with the workflow already established in your laboratory.See figures
Applications
Rotor-Gene AssayManager enables fully automated analysis of validated QIAGEN IVD assays (in preparation) or user defined tests using Rotor-Gene Q and QIAsymphony RGQ instruments in routine testing laboratories.
Supporting data and figures
Easy login and assay selection.
Simply log in to Rotor-Gene AssayManager and select the type of assay you want to run.
Resources
Instrument User Manuals (6)
Safety Data Sheets (1)
Protocol Files (1)
Certificates of Analysis (1)