Products
ipsogen BCR-ABL1 Mbcr IS-MMR Kits CE are intended for in vitro diagnostic use in Europe.

ipsogen BCR-ABL1 Mbcr IS-MMR DX Kit (24) CE
Cat. No. / ID: 670823
For 24 reactions: Reverse transcriptase, 5x RT buffer, dNTP mix, Random primers, RNase Inhibitor, DTT, High Positive RNA Control, IS-MMR Calibrator, Mbcr and ABL Single Plasmid Standards, Primers and Probe Mix ABL, Primers and Probe Mix BCR-ABL Mbcr Fusion Gene, qPCR Master mix, ROX II fluorescent dye
Features
- Results on the international scale (IS) and MMR reporting
- One IS-MMR calibrator per run to prevent drifting
- High reliability using EAC technology and single plasmid standards
- Compliance with EU IVD Directive 98/79/EC
- Easy workflow with ready-to-use solutions
Product Details
ipsogen BCR-ABL1 Mbcr IS-MMR Kits are in vitro diagnostic kits for real-time PCR on the Rotor-Gene Q and other real-time PCR instruments. The kits provide reagents optimized for reliable and sensitive detection and quantification of BCR-ABL p210 b2a2 or b3a2 transcripts in bone marrow or peripheral blood samples of acute lymphoblastic leukemia (ALL) or chronic myeloid leukemia (CML) patients previously diagnosed with a BCR-ABL Mbcr fusion gene event. The test is intended to evaluate the level of molecular response; results can be used for minimal residual disease (MRD) follow-up.
Performance
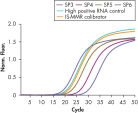
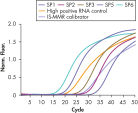
To ensure highest sensitivity, ipsogen BCR-ABL1 Mbcr IS-MMR Kits have been optimized to detect low levels of BCR-ABL p210 b2a2 or b3a2 transcripts (see figures Accurate detection of Mbcr plasmid standards, high positive RNA control, and IS-MMR calibrator and Reliable detection of ABL plasmid standards, high positive RNA control, and IS-MMR calibrator). The sensitivity has a limit of detection (LOD) of 0.0069.
Principle
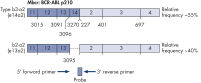
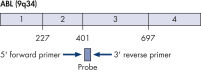
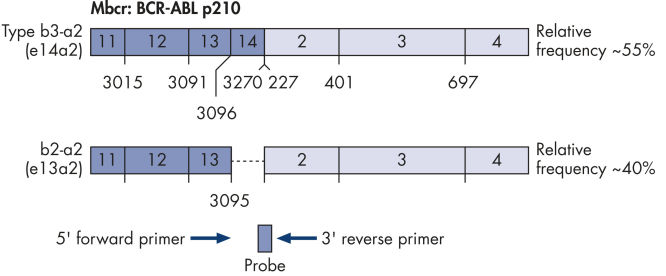
ipsogen BCR-ABL1 Mbcr IS-MMR Kits are ready-to-use kits for the detection of BCR-ABL p210 b2a2 or b3a2 transcripts using real-time PCR. The kits are based on the amplification and detection of specific BCR-ABL p210 b2a2 or b3a2 transcripts, relative to ABL control gene expression, in total RNA extracted from bone marrow or peripheral blood samples of acute lymphoblastic leukemia (ALL) or chronic myeloid leukemia (CML) patients previously diagnosed with a BCR-ABL Mbcr fusion gene event. The kits provide high levels of specificity, sensitivity, and reproducibility. Each ipsogen BCR-ABL1 Mbcr IS-MMR Kit provides 4 standard dilutions for ABL and 5 standard dilutions for Mbcr. Use of a single plasmid for BCR-ABL1 and ABL enables reproducible quantification of transcripts and limits variability. Primers and probe sets are designed according to Europe Against Cancer (EAC) recommendations (see figures Single plasmid for BCR-ABL gene transcript and ABL control gene transcript). The kits also include an IS-MMR calibrator allowing conversion of results to the international scale for accurate measurement of molecular response and major molecular response (MMR) status reporting. A high positive control RNA is also included to monitor the reverse transcription and amplification steps of ABL and BCR-ABL Mbcr during transcript quantification. The ipsogen BCR-ABL1 Mbcr IS-MMR DX Kit includes reagents for reverse transcription-PCR and real-time PCR.
Procedure
The first step is reverse transcription of total RNA in a sample into cDNA. The second step is the amplification of cDNA by real-time PCR. Using ipsogen BCR-ABL1 Mbcr IS-MMR Kits allows detection and quantification of BCR-ABL p210 b2a2 or b3a2 and ABL transcripts. Simply start the reaction using the optimized protocols described in the kit handbook.
Applications
The ipsogen BCR-ABL1 Mbcr IS-MMR Kits enable sensitive and reliable detection and quantification of BCR-ABL p210 b2a2 or b3a2 transcripts, conversion of results to the international scale, and MMR reporting, for in vitro diagnostic use.
Supporting data and figures
Single plasmid for BCR-ABL gene transcript.
Single plasmid for BCR-ABL gene transcript.
Resources
Kit Handbooks (2)
Safety Data Sheets (3)
Certificates of Analysis (1)