✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 33903
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- No hay necesidad de invertir tiempo en procedimientos de subclonación
- Se obtienen modificaciones postraslacionales en células de insectos o mamíferos
- Un constructo proporciona expresión eficaz en tres sistemas de expresión
Product Details
Performance
See figures
Principle
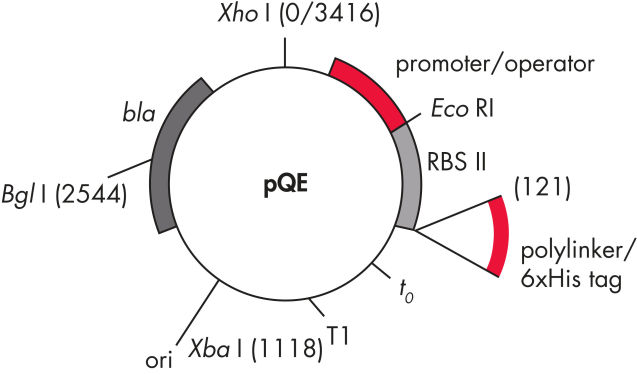
Los QIAexpress pQE Vectors aúnan un potente promotor T5 de fago (reconocido mediante ARN polimerasa de E. coli) con un módulo doble de represión de operón lac para proporcionar una expresión estrictamente regulada de alto nivel de proteínas recombinantes en E. coli. La síntesis de proteínas se bloquea de manera eficaz en presencia de niveles altos de represor lac y se mejora la estabilidad de constructos citotóxicos. Los vectores pQE (consulte la tabla y la figura Vectores pQE) facilitan la colocación de etiquetas 6xHis en el extremo N o C de la proteína recombinante.
| Elemento | Descripción |
| 1. Elemento promotor/operador optimizado |
Está compuesto por el promotor T5 de fago y dos secuencias de operón lac, que aumentan la probabilidad de unión del represor lac y aseguran la represión eficaz del potente promotor T5 |
| 2. Sitio sintético de unión al ribosoma RBSII | Para traducción eficaz |
| 3. Secuencia de codificación de etiqueta His | 5' o 3' a la región de clonación del polilinker |
| 4. Codones de parada traslacionales | En todos los marcos de lectura para preparar cómodamente los constructos de expresión |
| 5. Dos terminadores transcripcionales fuertes, |
t0 de fago lambda y T1 del operón rrnB de E. coli, para evitar la transcripción de la lectura y garantizar la estabilidad del constructo de expresión |
|
6. Origen de replicación ColE1 |
De pBR322 |
| 7. Gen beta-lactamasa (bla) | Confiere resistencia a la ampicilina |
See figures
Procedure
Los insertos que codifican proteínas de interés se clonan en constructos apropiados y se transforman en una cepa E. coli adecuada para su expresión. La expresión se induce añadiendo IPTG. Los constructos del pQE-TriSystem Vector pueden transformarse en E. coli, utilizarse como vector lanzadera para la expresión de proteínas recombinantes en células de insecto o transfectarse en células de mamífero.
Applications
El QIAexpress Expression System proporciona un alto nivel de expresión de proteínas idóneo
para muchas aplicaciones, como por ejemplo:
- Purificación de proteínas funcionales con configuración activa
- Purificación en condiciones desnaturalizantes para la producción de anticuerpos
- Cristalización para la determinación de estructuras tridimensionales
- Ensayos que implican interacciones proteína-proteína y proteína-ADN
Supporting data and figures
Vectores pQE.
Elementos numerados en la tabla.
Specifications
| Features | Specifications |
|---|---|
| In-frame cloning necessary | |
| Expression | |
| Tag removal sequence | |
| Expression species | |
| Tag | |
| N- or C-terminal tag | |
| All three reading frames provided |