Products

artus HI Virus-1 QS-RGQ Kit (24) CE
Cat. No. / ID. / ID. 4513363

artus HI Virus-1 RG RT-PCR Kit (24) CE
Cat. No. / ID. / ID. 4513263

artus HI Virus-1 RG RT-PCR Kit (96) CE
Cat. No. / ID. / ID. 4513265
Features
- Complete CE-IVD-compliant workflow from sample to detection
- High reliability using the internal control
- Highly sensitive detection of as few as 76.4 IU/ml
- Specific detection of HIV-1 genotypes A to H
- Accurate quantitation of viral load over a very broad range
Product Details
Performance
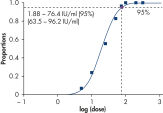
To ensure highest sensitivity, artus HI Virus-1 Kits have been optimized to detect low numbers of HIV-1 RNA. The analytical sensitivity of the artus HI Virus-1 QS-RGQ Kits is 76.4 IU/ml in consideration of the purification and assay setup using the QIAsymphony RGQ system (see figure " Highly sensitive detection of HIV-1 RNA"). (1 IU/ml corresponds to 0.45 copies/ml for detection of HIV-1 RNA on the Rotor-Gene Q. The conversion factor is an approximation based on an average factor across the assay's dynamic range.)
For highest specificity, validation of the artus HI Virus-1 Kits was carried out using various HIV-1 isolates, including all group M subtypes A–H, and related pathogens.
| Kit | artus HI Virus-1 RG RT-PCR Kit | artus HI Virus-1 QS-RGQ Kit |
|---|---|---|
| Validated sample type | Human EDTA plasma | Human EDTA plasma |
| Analytical sensitivity | 66.9 IU/ml | 76.4 IU/ml (corresponding to 34.4 copies/ml) |
| Linear range | 120 to >1 x 108 IU/ml | 1 x 102 to 1 x 108 IU/ml (corresponding to 4.5 x 101 to 4.5 x 107 copies/ml) |
| Specificity | HIV-1, including all group M subtypes A–H | HIV-1, including all group M subtypes A–H |
See figures
Principle
The artus HI Virus-1 RG RT-PCR Kit and the artus HI Virus-1 QS-RGQ Kit are based on the amplification and simultaneous detection of a specific region of the HIV-1 genome using real-time RT-PCR. The kits provide high levels of specificity, sensitivity (see figure " Highly sensitive detection of HIV-1 RNA"), and reproducibility over a broad linear range.
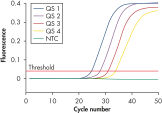
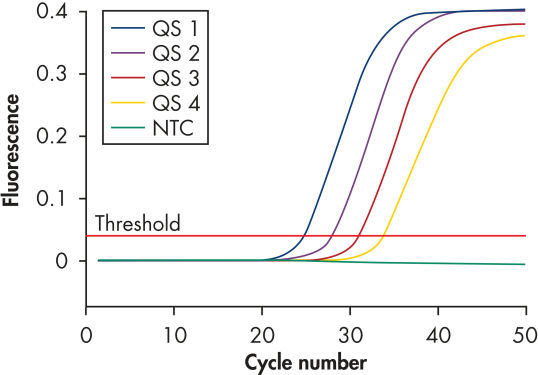
Each artus HI Virus-1 Kit provides 4 HIV-1 quantitation standards (see figure " Reliable quantitation of HIV-1 load"). Use of the standards enables accurate quantitation of viral load. In addition, the kits contains a second heterologous amplification system to identify possible PCR inhibition. This is detected as an internal control (IC) in a different fluorescence channel from the analytical PCR. The detection limit of the analytical HIV-1 PCR is not reduced.
| Kit | artus HI Virus-1 RG RT-PCR Kit and artus HI Virus-1 QS-RGQ Kit |
|---|---|
| Validated sample type | Human EDTA plasma |
| Amplicon | 93 nt region of the 5' LTR |
See figures
Procedure
artus HI Virus-1 RT-PCR Kits provide all necessary reagents optimized for reliable HIV-1 RNA detection and quantitation. Simply add template RNA to the ready-to-use PCR master mix, and start the reaction on the appropriate real-time cycler using the optimized cycling program described in the kit handbook.
Complete automated system from sample to HIV-1 detection
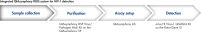
The QIAsymphony RGQ workflow solution for HIV-1 detection comprises the QIAsymphony SP for sample preparation, the QIAsymphony AS for assay setup, and the artus HI Virus-1 QS-RGQ Kit on the Rotor-Gene Q. The system enables reliable pathogen detection with a complete CE-IVD-compliant workflow (see figure " Integrated QIAsymphony RGQ system for HIV-1 detection").
Recommendations for manual viral RNA purification
The artus HI Virus-1 RG RT-PCR Kit is validated for use with viral RNA purified from human plasma using the CE-marked QIAamp DSP Virus Kit.
See figures
Applications
The artus HI Virus-1 RG RT-PCR Kit enables rapid and sensitive detection and quantitation of HIV-1 RNA purified from human plasma using the QIAamp DSP Virus Kit.
The artus HI Virus-1 QS-RGQ Kit is designed to be used with the QIAsymphony RGQ system, providing a complete CE-IVD-compliant workflow from sample to HIV-1 RNA detection and quantitation.
Supporting data and figures
Reliable quantitation of HIV-1 load.
Specifications
| Features | Specifications |
|---|---|
| Quantitative/qualitative | Quantitative |
| What detected | Human Immunodeficiency Virus type 1 RNA |
| RUO/CE/ASR | CE |
| Sample type | EDTA plasma |
| Recommended sample prep | QIAamp DSP Virus Kit |
| Thermal cycler | Rotor-Gene 6000 |