RT2 SYBR® Green qPCR Mastermixes
リアルタイムPCRアプリケーション用
リアルタイムPCRアプリケーション用
Cat. No. / ID. / ID. 330513

Cat. No. / ID. / ID. 330502

Cat. No. / ID. / ID. 330523

Cat. No. / ID. / ID. 330500

Cat. No. / ID. / ID. 330529

Cat. No. / ID. / ID. 330512

Cat. No. / ID. / ID. 330503

Cat. No. / ID. / ID. 330524

Cat. No. / ID. / ID. 330510

Cat. No. / ID. / ID. 330509

Cat. No. / ID. / ID. 330521

Cat. No. / ID. / ID. 330501

Cat. No. / ID. / ID. 330519

Cat. No. / ID. / ID. 330514

Cat. No. / ID. / ID. 330522

Cat. No. / ID. / ID. 330511

Cat. No. / ID. / ID. 330504

Cat. No. / ID. / ID. 330520
RT² SYBR Green qPCR Mastermixesには、RT2 qPCR Primer AssayおよびRT2 Profiler PCR Arrayを用いたSYBR GreenベースのリアルタイムPCRに必要な至適化済み試薬とバッファーがすべて入っていて、ほとんどのリアルタイムPCR装置で使用できます。
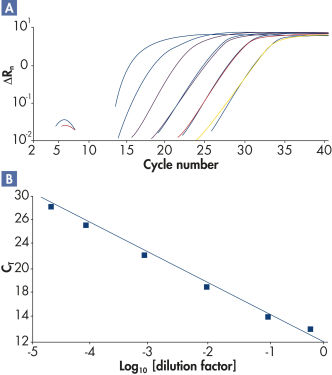
RT² SYBR Green qPCR Mastermixesを用いて行なった高性能リアルタイムPCRは、高い増幅効率(図“ 幅広いダイナミックレンジで高い増幅効率”)および高レベルの感度と特異性(図“ ポリメラーゼ活性の厳密な制御により高い特異性を実現”)を実証しています。
RT2 SYBR Green qPCR Mastermixesには、リアルタイムPCRバッファー、高性能HotStart DNA Taq polymerase、ヌクレオチド、SYBR Green色素が入っています。装置の光学系を最適化するために、フルオレセインまたはROX色素のいずれかが入ったマスターミックスもあります。化学修飾され厳密にコントロールされたHotStart酵素を用いることにより、プライマーダイマーあるいは非特異的な産物の増幅を回避でき、正確なSYBR Green結果が得られます。
RT2 SYBR Green qPCR Mastermixは reference dyeが不要な装置でのSYBR Greenベースの検出用リアルタイムPCRアプリケーションに最適です。 対象装置: Bio-Rad models CFX96、CFX384; Bio-Rad/MJ Research Chromo4、DNA Engine Opticon、DNA Engine Opticon 2; Roche LightCycler 480 (96-well and 384-well); Eppendorf Mastercycler ep realplex without ROX filter set; Cepheid SmartCycler。
RT2 SYBR Green Fluor qPCR Mastermixは、 reference dyeとしてフルオレセインを使用する装置でのSYBR Greenベースの検出用リアルタイムPCRアプリケーションに最適です。 対象装置:Bio-Rad models iCycler、iQ5、MyiQ、MyiQ2。
RT2 SYBR Green ROX qPCR Mastermixは、 reference dyeとしてROXを使用する装置でのSYBR Greenベースの検出用リアルタイムPCRアプリケーションに最適です。 対象装置:QIAGENの Rotor-Gene Q; Applied Biosystems models 5700, 7000, 7300, 7500 (Standard and Fast), 7700, 7900HT (Standard and Fast 96-well block, 384-well block), StepOnePlus, ViiA 7 (Standard and Fast 96-well block, 384-well block); Eppendorf Mastercycler ep realplex with or without ROX filter set; Stratagene models Mx3000P, Mx3005P, Mx4000;Takara TP-800。