Products
References Kotton, C.N. et al. (2013) Transplantation 96, 333. Kumar, D. et al. (2009) Am. J. Transplant. 9, 1214. Walker, S. et al. (2007) Transplant. Infect. Dis. 9, 165. Westall, G.P. et al. (2008) Am. J. Transplant. 8, 1749. QuantiFERON-CMV Package Insert, December 2012, 1075110 Rev. 01. Fleming, T. et al. (2010) J. Med. Virol. 82, 433. Lisboa, L.F. et al. (2012) Am. J. Transplant. 93, 195. Weseslindtner, L. et al. (2012) Am. J. Transplant. 12, 2172. Manuel, O. et al. (2013) Clin. Infect. Dis. 56(6), 817.
QuantiFERON-CMV Kits are intended for in vitro diagnostic use in Europe.
Features
- Predict risk of new and recurrent CMV disease (1)
- Monitor transplant patient's level of anti-CMV immunity (2)
- Determine both pre- and posttransplant CMV-risk stratification (1–4)
- Guide therapeutic decision making (1)
Product Details
QuantiFERON-CMV is an in vitro diagnostic test using a peptide cocktail simulating human CMV proteins to stimulate cells in heparinized whole blood (5). Detection of IFNγ responses by ELISA may indicate CMV immunity.
Performance
QuantiFERON-CMV is an in vitro diagnostic test using a peptide cocktail simulating human CMV proteins to stimulate cells in heparinized whole blood. Detection of interferon-gamma (IFNγ) by enzyme-linked immunosorbent assay (ELISA) is used to identify in vitro responses to these peptide antigens that are associated with CMV infection.(1, 5) Loss of this immune function may be associated with development of CMV disease.
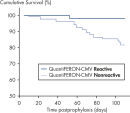
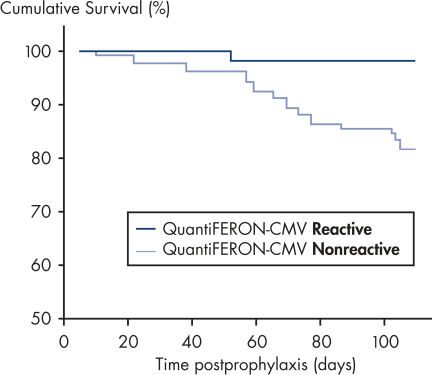
In a posttransplant setting, patients who have cellular immune response to CMV at the end of prophylaxis have a significantly lower risk of developing CMV disease than those who do not have a detectable immune response. This indicates that QuantiFERON-CMV may predict the development of late-onset CMV disease in transplant recipients (2, 4, 6–9)(see figure " Time to development of CMV disease in patients at the end of prophyplaxis").
In a posttransplant setting, patients who have cellular immune response to CMV at the end of prophylaxis have a significantly lower risk of developing CMV disease than those who do not have a detectable immune response. This indicates that QuantiFERON-CMV may predict the development of late-onset CMV disease in transplant recipients (2, 4, 6–9)(see figure " Time to development of CMV disease in patients at the end of prophyplaxis").
See figures
Principle
QuantiFERON-CMV uses 3 specialized blood collection tubes (Nil, CMV Antigen, Mitogen). The CMV Antigen tubes are coated with peptides that simulate CD8+ specific epitopes of CMV proteins. Stimulation of a patient's T cells with CMV peptides results in the production of IFNγ in infected individuals. A robust IFNγ response in the CMV Antigen tube is indicative of immunity to CMV (see figure " A theorhetical model of QuantiFERON-CMV responses").
See figures
Procedure
Patient blood is collected by venipuncture directly into 3 blood collection tubes (Nil, CMV Antigen, Mitogen) and then incubated for 16 to 24 hours at 37°C. After incubation, plasma is harvested and the ELISA assay is performed to detect human IFNγ, using a standard curve and measurement of optical density. Calculations and test interpretation may be performed manually. QuantiFERON-CMV Analysis software is also available.
Applications
QuantiFERON-CMV is used to assess the level of CMV-specific immunity in patients at risk of developing CMV disease. QuantiFERON-CMV may assist clinicians to predict the risk of new and recurrent CMV disease, guide therapeutic decision-making, and improve patient health (1).
Supporting data and figures
Time to development of CMV disease in patients at the end of prophylaxis.
In a posttransplant setting, patients with a reactive QuantiFERON-CMV test remain free from CMV disease significantly more often, and for longer, than patients with a nonreactive QuantiFERON-CMV test after the cessation of antiviral prophylaxis. This indicates QuantiFERON-CMV may predict development of late-onset CMV in transplant recipients (2).
Resources
Kit Handbooks (1)
Safety Data Sheets (1)
Certificates of Analysis (1)