✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Effectene Transfection Reagent (1 ml)
Cat. No. / ID: 301425
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- High efficiency in the presence of serum
- Efficient transfection with low DNA amounts
- Far lower cytotoxicity and gentler than many alternatives
- Suitable for high-throughput screening
Product Details
Performance
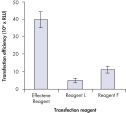
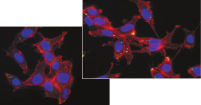
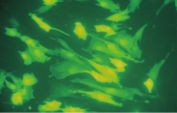
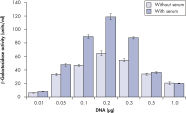
Higher transfection efficiencies of plasmid DNA are achieved with Effectene Transfection Reagent than with other reagents when used with the recommended procedures (see figure " High transfection efficiencies using Effectene Reagent"). Effectene Transfection Reagent is suitable for transfecting sensitive cell lines with oligonucleotides (see figure " Transfection of oligonucleotides using Effectene Reagent") and is particularly effective for primary cells (see figure " 40% transfection efficiency in primary cells"). Many cell lines and primary cells have been successfully transfected using Effectene Reagent; cell type-specific transfection protocols are available. Cytotoxicity is minimal because transfection with Effectene Reagent can be performed in the presence of serum and requires low amounts of DNA (see figure " Serum and DNA quantity vs. transfection efficiency").
The application of recombinant DNA technology to fields such as drug discovery and development has led to an increased need for high-throughput transfection. Transfection using Effectene Transfection Reagent requires low amounts of DNA and minimal handling. In addition, removal of transfection complexes is not required, making this reagent highly suitable for high-throughput screening.
See figures
Principle
Effectene Transfection Reagent is an innovative non-liposomal lipid formulation that is used in conjunction with a special DNA-condensing enhancer and optimized buffer to achieve high transfection efficiencies. The enhancer first condenses the DNA molecules and Effectene Reagent subsequently coats them with cationic lipids providing a particularly efficient way of transferring DNA into eukaryotic cells. This feature ensures excellent reproducibility of transfection complex formation.
Procedure
The Effectene procedure has two steps. DNA is first mixed with Enhancer and a buffer that provides optimal salt conditions for efficient DNA condensation. This step requires just 2–5 minutes. Effectene Reagent is then added and the mixture is incubated for 5–10 minutes to allow Effectene–DNA complexes to form. The complexes are mixed with growth medium (which can contain serum and antibiotics), and added directly to the cells. The cells are then incubated until harvested and analyzed for gene expression (see flowchart " Effectene transfection procedure").
See figures
Applications
Effectene Transfection Reagent is suitable for transient and stable transfection of a broad range of cell types.
Supporting data and figures
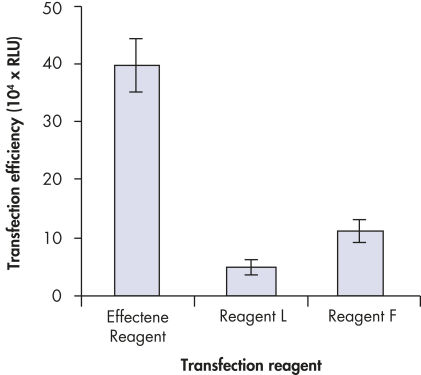
High transfection efficiencies using Effectene Reagent.
Specifications
| Features | Specifications |
|---|---|
| Applications | Plasmid transfection, protein overexpression, reporter studies |
| Technology | Non-liposomal lipid formulation in conjonction with a DNA-condensing enhancer |
| Number of possible transfections | 160 transfections in 12-well plates / 1 ml reagent |
| Cell type | Eukaryotic cells (primary cells and sensitive cells) |
| Features | Non-liposomal lipid formulation, minimal cytotoxicity |
| Transfection type | Transient and stable transfection |
| Controls | Not included |
| Nucleic acid | DNA |