QIAseq FX Single Cell RNA Library Kit
Single cell RNA-seq libraries that provide a deeper view of the transcriptome
Single cell RNA-seq libraries that provide a deeper view of the transcriptome
The QIAseq FX Single Cell RNA Library kit is an end-to-end library preparation solution for RNA-seq from single cells or low amounts of RNA. The kit includes all reagents required for cell lysis, reverse transcription, cDNA amplification and PCR-free NGS library preparation. It produces high-quality NGS libraries and generates a tube of excess amplified cDNA that can be stored for follow-up experiments. The kit is ideally suited for transcript discovery and differential expression from single eukaryotic cells and RNA-seq from limited samples including viral RNA.
Need a quote for your research project or would you like to discuss your project with our specialist team? Contact Us
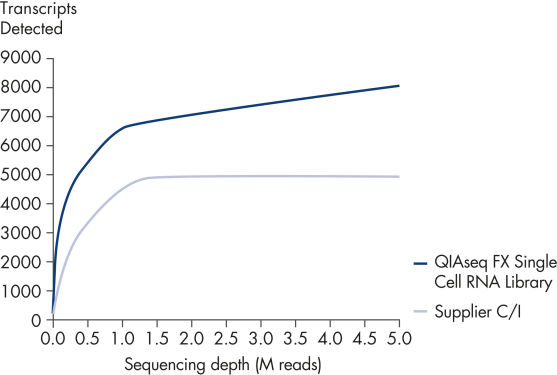
The kit detects a greater number of transcripts than competing methods at any sequencing depth. In some single-cell RNA-seq workflows PCR is used extensively for both cDNA amplification and to amplify the minute amounts of library produced. This has a negative effect on library diversity from the loss of low-abundance transcripts due to both stochastic effects and PCR-bias during cDNA amplification and the generation of PCR duplicates during library amplification. Additionally, these PCR systems often introduce GC-bias and length-bias, which can make the sequencing of GC-rich or long transcripts difficult. In contrast, the QIAseq FX Single Cell RNA Library Kit relies on a highly-efficient MDA reaction to amplify cDNA, and generates enough material that library amplification is not necessary, delivering a completely PCR-free workflow. This eliminates PCR duplicates and maximizes the number of transcripts detected, including long transcripts such as lncRNAs.
The QIAseq FX Single Cell RNA Library Kit is a complete cell-to-library solution that generates RNA-seq libraries from single eukaryotic cells or from pg-levels of purified RNA. The kit contains reagents for the efficient lysis of isolated single cells from common cell-sorting or cell-isolation platforms. After lysis, genomic DNA is degraded and reverse transcription is performed. This reaction can be primed from either an included oligo-dT primer in order to reduce the number of reads arising from ribosomal RNA and to enrich for polyadenylated RNAs, or from random primers, for example when sequencing viral RNA genomes from limited samples. After reverse transcription and second strand synthesis, a ligation step generates a long, double-stranded cDNA template which is amplified via multiple displacement annealing using a specially engineered, ultra-high fidelity phi29 polymerase. This generates µg amounts of amplified cDNA, some of which can be stored for later use or confirmatory testing. Amplified cDNA is fragmented with a highly random, enzymatic fragmentation step, which generates inserts compatible with common sequencing read lengths, and insert size can be varied according to individual preferences. No exact quantification of the cDNA is necessary prior to fragmentation, as both the amplification process and fragmentation reaction are extremely robust. Insert DNA is end-polished and subjected to a highly efficient ligation reaction with the included barcoded adapters, which can be purified by standard methods prior to quantification and sequencing. Amplification of the library with PCR is not necessary, so the production of PCR duplicates is avoided and library diversity remains high.