QIAcuity One, 2plex Device
Cat. No. / ID: 911001
Features
- 완전 통합 시스템
- 확장 가능 형식(1개, 4개, 8개 플레이트 기기)
- 고급 멀티플렉싱 기능(최대 5 플렉스)
- 유연성 있는 샘플 처리량
- 약 2시간 내에 포괄적인 결과 산출
Product Details
QIAcuity Digital PCR System은 돌연변이 검출, 복제 수 변이(CNV), 유전자 발현 연구, 유전자 편집 분석 등에 대한 정밀하고 멀티플렉싱된 정량화 결과를 제공하도록 설계되었습니다. 이 나노플레이트 기반 시스템은 분획, 열순환(thermocycling) 및 이미징의 표준 dPCR 워크플로우를 최소한의 직접 작업 시간으로 자동화된 플랫폼에 완벽하게 통합합니다.
이 시스템은 QIAcuity Nanoplates 및 액세서리와 함께 사용합니다.
가상 데모를 통해 QIAcuity에 대해 더 자세히 알아보세요.
Performance
QIAcuity Digital PCR System은 모든 실험실에서 절대 정량화를 합리적인 비용으로 할 수 있게 합니다. 워크어웨이 자동화는 최소한의 작업 시간으로 분획, 열순환(thermocycling) 및 이미징의 전체 디지털 PCR 워크플로우를 하나의 기기로 통합하여 간소화합니다. 또한 현재 qPCR 분석 항목을 QIAcuity Digital PCR System에 쉽게 적용할 수 있습니다. qPCR에서 전환할 때 플레이트 사용 방식에는 변화가 필요 없으므로 약 2시간 내에 빠른 분석 항목 설정과 빠른 결과를 얻을 수 있습니다.
QIAcuity 기기 – 기능 및 사양
| 기능 | QIAcuity One | QIAcuity Four | QIAcuity Eight |
|---|---|---|---|
| 처리 플레이트 | 1 | 4 | 8 |
| 검출 채널(멀티플렉싱) | 2 또는 5 | 5 | 5 |
| 서모사이클러 | 1 | 1 | 2 |
| 결과까지 걸리는 시간 | 약 2시간 |
첫 번째 플레이트는 약 2시간 그다음 플레이트당 ~60분 |
첫 번째 플레이트는 약 2시간 그다음 플레이트당 ~30분 |
| 처리량(작업일 하루에 처리하는 검체) |
최대 384개(96웰) 최대 96개(24웰) |
최대 672개(96웰) 최대 168개(24웰) |
최대 1248개(96웰) 최대 312개(24웰) |
Principle
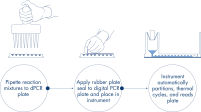
피펫팅 및 로딩, 실험 실행, 결과 분석의 간단한 3단계로 약 2시간 내에 원하는 dPCR 결과를 얻을 수 있습니다.
나노플레이트에서의 dPCR 반응 원리는 여기에 설명되어 있습니다.
Procedure
qPCR 실험과 마찬가지로 샘플 준비에는 마스터 믹스, 프로브 및 프라이머를 96웰 또는 24웰 나노플레이트로 옮긴 다음 샘플을 추가하는 작업이 포함됩니다. 이 시스템은 분획, 열순환(thermocycling) 및 이미징을 하나의 완전 자동화 기기로 통합하여 사용자가 2시간 이내에 샘플에서부터 결과를 얻을 수 있습니다. Suite 소프트웨어에서 분석을 수행할 수 있으며, 표적 서열의 마이크로리터당 복제 농도뿐만 아니라 양성 검체나 NTC와 같은 품질 관리도 제공됩니다. 이 분석은 또한 동일한 LAN(근거리 통신망) 내의 원격 컴퓨터로 확장될 수 있습니다.
Applications
QIAcuity 기기는 QIAcuity Nanoplates 및 QIAcuity PCR 키트와 함께 다음을 포함한 디지털 PCR 애플리케이션을 지원합니다.
- 희귀 돌연변이 검출
- 복제 수 변이 분석
- 유전자 발현 분석
- 병원체 검출
- 유전형 분석
- miRNA 연구
Software
기기와 함께 제공되고 별도의 컴퓨터에 설치된 QIAcuity Software Suite는 한 대의 기기에 직접 연결하거나 기존 LAN(근거리 통신망)을 사용하여 한 대 이상의 QIAcuity 기기를 제어합니다. QIAcuity Software Suite를 사용하여 디지털 PCR 실험, 샘플 및 반응 혼합물을 정의하고 Nanoplate에 배정하고 QIAcuity 기기로 전송할 수 있습니다. 실행 후 데이터를 분석하고 보고서를 만들고 외부 분석을 위해 데이터를 내보낼 수 있습니다. 이 소프트웨어는 반복되는 플레이트 레이아웃 또는 플레이트 실행 매개변수에 쉽게 액세스할 수 있도록 하는 여러 템플릿 기능을 제공하여 디지털 PCR 경험을 더욱 향상합니다.
LAN에 통합되면 컴퓨터가 LAN을 통해 클라이언트 역할을 하는 다른 컴퓨터에 액세스할 수 있는 서버로서 QIAcuity Software Suite 기능을 호스팅합니다. 이를 통해 여러 사용자가 여러 컴퓨터에 소프트웨어를 설치하거나 인터넷 연결을 통해 데이터에 액세스하고 교환할 필요 없이 다른 방이나 사무실에서 소프트웨어에 액세스하고 표준 브라우저를 통해 데이터를 분석할 수 있습니다.
Services
QIAGEN의 다양한 서비스 솔루션으로 기기를 관리하십시오. 귀하의 필요성에 맞는 특정 서비스 계약에 대해 알아보십시오.
Supporting data and figures
간단하고 신속한 플레이트 기반 워크플로우