✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
ipsogen BCR-ABL1 mbcr Kit (24) CE
Cat. No. / ID: 670023
For 24 reactions: ABL Control Gene Standards, BCR-ABL mbcr Fusion Gene Standards, Primers and Probe Mix ABL, Primers and Probe Mix BCR-ABL mbcr Fusion Gene
Log in To see your account pricing.
The ipsogen BCR-ABL1 mbcr Kit CE is intended for in vitro diagnostic use in Europe.
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Highly accurate and sensitive quantitative assay
- EAC standardized assay for reproducible results
- Compliance with EU IVD Directive 98/79/EC
- Easy workflow with ready-to-use solutions
Product Details
The ipsogen BCR-ABL1 mbcr Kit is an in vitro molecular diagnostic kit for real-time PCR on the Rotor-Gene Q and other real-time PCR instruments. The kit provides reagents optimized for reliable and sensitive detection and quantification of BCR-ABL mbcr p190 e1a2 transcripts in bone marrow or peripheral blood samples of Ph-positive acute lymphoblastic leukemia (ALL) patients previously diagnosed with a BCR-ABL mbcr fusion gene event. The test is intended to monitor efficacy of treatment in patients undergoing therapy and for minimal residual disease (MRD) follow-up to monitor disease relapse.
Performance
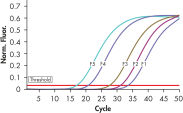
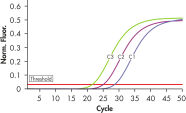
To ensure highest sensitivity, the ipsogen BCR-ABL1 mbcr Kit has been optimized to detect BCR-ABL mbcr p190 e1a2 and ABL transcripts and uses prediluted plasmid standards and primers and probe mixes (see figures Accurate detection of BCR-ABL mbcr plasmid standards and Reliable detection of ABL plasmid standards).
Principle
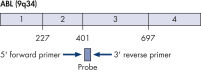
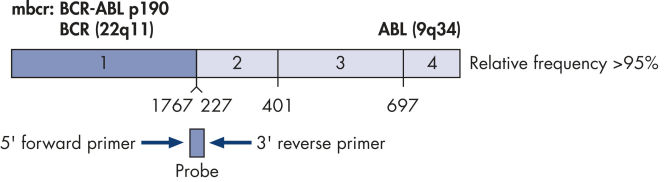
The ipsogen BCR-ABL1 mbcr Kit is a ready-to-use kit for the detection of BCR-ABL mbcr p190 e1a2 fusion gene transcripts using real-time PCR. The kit is based on the amplification and detection of specific BCR-ABL p190 e1a2 transcripts, relative to ABL control gene expression, in total RNA extracted from bone marrow or peripheral blood samples of Ph-positive acute lymphoblastic leukemia (ALL) patients previously diagnosed with a BCR-ABL mbcr fusion gene event (see figures BCR-ABL mbcr fusion gene transcript and ABL control gene transcript). The kit provides high levels of specificity, sensitivity, and reproducibility. The ipsogen BCR-ABL1 mbcr Kit provides 5 standard dilutions for BCR-ABL mbcr and 3 standard dilutions for ABL. Use of the standards enables accurate quantification of transcripts. The technology used for quantification of BCR-ABL mbcr has been standardized according to Europe Against Cancer (EAC) recommendations and the ipsogen BCR-ABL1 mbcr Kit uses this validated technology to calibrate results.
Procedure
The first step is reverse transcription of total RNA in a sample into cDNA. The second step is the amplification of cDNA by real-time PCR. Using the ipsogen BCR-ABL1 mbcr Kit allows detection and quantification of BCR-ABL mbcr p190 e1a2 and ABL transcripts. Simply start the reaction using the optimized protocols described in the kit handbook.
Applications
The ipsogen BCR-ABL1 mbcr Kit enable sensitive and reliable detection and quantification of BCR-ABL mbcr p190 e1a2 transcripts, relative to ABL control gene expression, for in vitro diagnostic use.
Supporting data and figures
BCR-ABL mbcr fusion gene transcript.
BCR-ABL mbcr fusion gene transcript.
Resources
Kit Handbooks (1)
Safety Data Sheets (2)
Certificates of Analysis (1)