QIAcuity One, 2plex Device
Cat no. / ID. 911001
Features
- Fully integrated system
- Scalable format (1-, 4- and 8-plate instruments)
- Advanced multiplexing capabilities (up to 12 plex)
- Up to six standard channels plus two hybrid channels for LSS dyes
- Flexible sample throughput
- Comprehensive results in approx. 2 hours
Product Details
The QIAcuity Digital PCR System is designed to deliver precise and multiplexed quantification results for mutation detection, copy number variation (CNV), gene expression studies, gene-editing analysis, and many more. This nanoplate-based system seamlessly integrates a standard dPCR workflow of partitioning, thermocycling and imaging into a walk-away automated platform with minimal hands-on time.
The system is used in conjunction with the QIAcuity nanoplates, reagents and assays.
Explore the virtual demo to learn more about QIAcuity.
Performance
The QIAcuity Digital PCR System makes absolute quantification accessible and affordable for all laboratories. The walk-away automation integrates and streamlines the entire digital PCR workflow of partitioning, thermocycling and imaging into a single instrument with minimal hands-on time. It is also easy to adapt your current qPCR assays to the QIAcuity Digital PCR System. No change in plate handling is required when coming from qPCR, assuring fast assay setup and quick results in approx. 2 hours.
QIAcuity Instruments – features and specifications
| Feature | QIAcuity One 2plex | QIAcuity One 5plex | QIAcuity Four | QIAcuity Eight |
|---|---|---|---|---|
| Plates processed | 1 | 1 | 4 | 8 |
| Detection channels | 2 | 81) (6+2 hybrid2) |
81) (6+2 hybrid2) |
81) (6+2 hybrid2) |
| Multiplexing capability | 4 | 123)1) | 123)1) | 123)1) |
| Thermocycler(s) | 1 | 1 | 1 | 2 |
| Time to result | Approx. 2 h | Approx. 2 h |
First plate approx. 2 h Every ∼80 min a following plate |
First plate approx. 2 h Every ∼40 min a following plate |
| Throughput (samples processed in a workday) |
Up to 384 (96-well) Up to 96 (24-well) |
Up to 384 Up to 96 |
Up to 672 (96-well) Up to 168 (24-well) |
Up to 1248 (96-well) Up to 312 (24-well) |
1) Requires use of QIAcuity High Multiplex Probe PCR Kit in case of multiplexing >5plex
2) Hybrid channels are used for Long Stokes Shift (LSS) dyes
3) Detection of 12 targets in parallel can be achieved by using amplitude multiplexing in the six standard channels. When combining hybrid channels for single plex LSS dyes and amplitude multiplexing, the total possible multiplex can be increased to 14. However, this combination is not recommended due to the optimization demand for all assays used in a reaction mix and their corresponding crosstalk compensation.
Principle
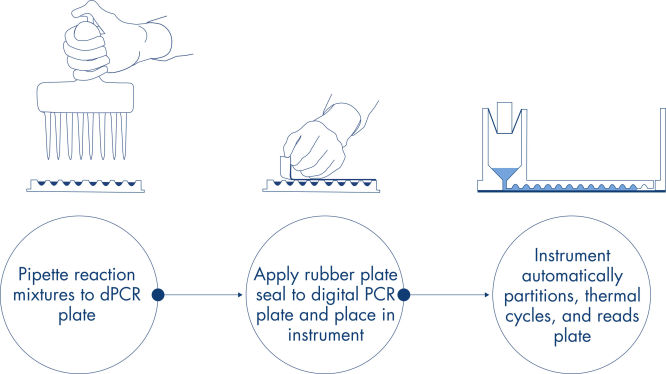
In just 3 simple steps, you can have the dPCR result you want in approx. 2 hours: pipette and load, run the experiment, analyze results.
The principle of the dPCR reaction in the nanoplates is described here.
Procedure
Just like in qPCR experiments, sample preparation includes the transfer of master mix, probes and primers to a 96- or 24-well nanoplate, followed by the addition of samples. The system integrates partitioning, thermocycling and imaging into a single fully automated instrument that takes users from the sample to result in under 2 hours. One can perform analysis on the Software Suite, providing the concentration in copies per microliter of your target sequence as well as for quality control such as positive samples or NTC. This analysis can also be extended to remote computers within the same local area network (LAN).
Applications
The QIAcuity instruments, together with the QIAcuity Nanoplates and the QIAcuity PCR kits, enable digital PCR applications, including:
- Rare mutation detection
- Copy number variation analysis
- Gene expression analysis
- Pathogen detection
- Genotyping
- miRNA research
- Cell and gene therapy
Software
The QIAcuity Software Suite provided with the instrument and installed on a separate computer controls one or multiple QIAcuity instruments, either connected directly to one instrument or using an existing local area network (LAN). Using the QIAcuity Software Suite, digital PCR experiments, samples and reaction mixes can be defined, assigned to Nanoplates, and transferred to the QIAcuity instrument. After the run, data can be analyzed, reports can be created and data can be exported for external analysis. The software provides multiple template functionalities to make repeating plate layouts or plate run parameters easily accessible, further transforming your digital PCR experience.
When integrated into a local area network, the computer is hosting the QIAcuity Software Suite function as a server that is accessible via LAN to other computers serving as clients. This enables multiple users to access the software from other rooms or offices and analyze data via a standard browser without the need to install the software on multiple computers or access and exchange data via internet connections.
Services
Protect your instrument with diverse service solutions from QIAGEN. Learn more about a specific service agreement to match your needs.
Supporting data and figures
A simple and rapid plate-based workflow
Kits & Consumables
Service Plans
QIAcuity One 2plex Core Agreement
Cat no. / ID. 9245358
QIAcuity One 5plex Core Agreement
Cat no. / ID. 9245359