Ni-NTA Agarose
用于通过重力流色谱法纯化 His 标签蛋白
用于通过重力流色谱法纯化 His 标签蛋白
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 30210
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Ni-NTA Agarose 是一种亲和层析基质,用于纯化带有 His 标签的重组蛋白。His 标签中的组氨酸残基凭借高特异性和亲和力,与固定镍离子配位球中的空位结合。澄清的细胞裂解物被加载到基质上。His 标签蛋白与基质结合,其他蛋白则穿过基质。洗涤后,在非变性或变性条件下在缓冲液中洗脱 His 标签蛋白。
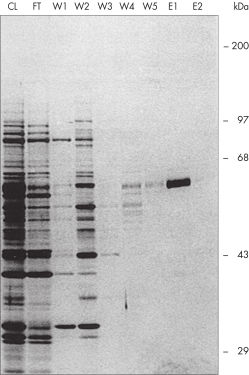
Ni-NTA Agarose 将 Ni-NTA 与 Sepharose CL-6B 支链耦合在一起,具有高结合能力,非特异性结合极少(见图 原生条件下一步纯化)。这种材料具有出色的处理性能,适用于大多数规模的间歇式和柱式纯化。Ni-NTA Agarose 既可单独提供,也可作为 QIAexpress Kits 的组成部分提供,这些试剂盒是用于从大肠杆菌中高效表达和纯化 His 标签蛋白的全套试剂盒。
QIAexpress Ni-NTA 蛋白纯化系统基于的是获得专利的 Ni-NTA(次氮基三乙酸镍)树脂对含有六个或更多组氨酸残基(连续或交替)的亲和标记(His 标记)的蛋白的显著选择性。该技术可在非变性或变性条件下,从任何表达系统中一步纯化几乎所有 His 标签蛋白。NTA 有四个镍离子螯合位点,与只有三个位点可与金属离子相互作用的金属螯合纯化系统相比,NTA 与镍的结合更紧密。额外的螯合位点可防止镍离子浸出,与其他金属螯合纯化系统相比,它的结合能力更强,制备的蛋白纯度更高。QIAexpress 系统可用于从任何表达系统中纯化 His 标签蛋白,包括杆状病毒、哺乳动物细胞、酵母和细菌。
| 变性剂 | 洗涤剂 | 还原剂 | 其他 | 盐 | 用于长期储存 |
|---|---|---|---|---|---|
| 6 M Gu·HCl | 2% Triton X-100 | 20 mM β-ME | 50% 甘油 | 4 M MgCl2 | 高达 30% 的乙醇 |
| 8 M 尿素 | 2% 吐温 20 | 10 mM DTT | 20% 乙醇 | 5 mM CaCl2 | 或 100 mM NaOH |
| 1% CHAPS | 20 mM TCEP | 20 mM 咪唑 | 2 M NaCl |
Ni-NTA 基质能可靠地一步纯化适用于以下任何应用的 His 标签蛋白:
| Features | Specifications |
|---|---|
| Applications | 蛋白质组学 |
| Tag | 6xHis 标签 |
| Bead size | 45–165 µm |
| Special feature | 批量和柱纯化 |
| FPLC | 否 |
| Processing | 手动 |
| Support/matrix | Sepharose CL-6B |
| Start material | 细胞裂解物 |
| Scale | 大规模 |
| Binding capacity | 最多 50 mg/ml |
| Gravity flow or spin column | 重力流 |
| Yield | 100 µg – 100 mg |