✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
QuantiTect Virus Kit (1000)
Cat. No. / ID: 211015
✓ 全天候自动处理在线订单
✓ 博学专业的产品和技术支持
✓ 快速可靠的(再)订购
特点
- 高灵敏度的单一和多重检测
- 在同一反应中检测病毒RNA和/或DNA
- 清晰检测弱阳性信号
- 通用的快速两步法实验方案
- 5x预混液灵敏度更高,起始样本量更大
产品详情
绩效
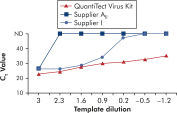
应用QuantiTect Virus Kits进行扩增可在广泛的稀释范围内获得陡峭的Sigma曲线,即使在模板量少、CT值高的情况下也是如此(参见" Unambiguous determination of CT values over a wide dynamic range")。由此可精确确定real-time PCR的CT值,定量分析病毒核酸。
多重分析可检测含有内参的多种病毒RNA和/或DNA靶分子,适用线性范围广泛,且不影响检测灵敏度(参见" Reliable detection of viral RNA over a wide linear range"和" Improved detection of low amounts of viral RNA")。
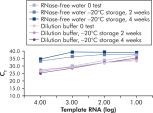
试剂盒中提供的QuantiTect Nucleic Acid Dilution Buffer可在稀释和反应体系构建过程中稳定RNA和DNA标准品,避免在试管和枪头等管壁丢失核酸。该缓冲液确保可靠稀释用于病毒核酸定量分析的标准核酸,可获得从低到高CT值的广泛线性范围。缓冲液可避免降解,延长标准品的储存时间(参见" Reliable dilution and storage of RNA standards")。
查看图表
原理
QuantiTect Virus Kit可通过单一或多重分析对病毒核酸高度灵敏的检测(参见" QIAGEN multiplex kits")。优化的预混液保证PCR产物在多重反应中的扩增效率和灵敏度与在单一反应中相同。
在同一反应液中扩增内参和靶基因,可减少手工误差,提高基因定量分析的可靠性。QuantiTect Virus Buffer含有浓度平衡的K+、NH4+和独特合成的MP因子,可促使引物和探针与核酸模板稳定、高效的退火,提高PCR效率(参见" Unique PCR buffer")。此外,Sensiscript Reverse Transcriptase确保对病毒RNA高灵敏度的逆转录,而HotStarTaq Plus DNA Polymerase具有严格的热启动,可防止非特异性产物的形成。
| 试剂盒组份 | 特点 | 优势 | |
|---|---|---|---|
| 5x QuantiTect Virus Master Mix | 浓缩的预混液 | 浓度高,经过优化,可灵敏的检测病毒核酸 | 起始样本量更大,灵敏度更高 |
| HotStarTaq Plus DNA Polymerase | 在95ºC下5分钟即可活化 | 室温下构建qPCR反应体系 | |
| QuantiTect Virus Buffer | NH4+和K+的平衡组合 | 特异性引物退火确保PCR结果可靠 | |
| 合成的Factor MP | 在单管内对4个基因进行可靠的多重分析 | ||
| 其他的试剂盒组份 | QuantiTect Virus RT Mix | Contains a unique formulation of Sensiscript Reverse Transcriptase | 优化用于高度灵敏检测病毒RNA |
| QuantiTect Nucleic Acid Dilution Buffer | Proprietary buffer formulation for dilution and storage of nucleic acid standards. | 在稀释和反应体系构建时稳定RNA和DNA标准,避免核酸在塑料表面(如指管或枪头)的损失 |
查看图表
程序
QuantiTect Virus Kit使用序列特异性探针可对病毒核酸(RNA和/或DNA)及内参进行高度灵敏的real-time PCR分析。反应可选择是否进行逆转录步骤,可灵活设计实验检测RNA、DNA或者RNA及DNA。选用使用手册中的实验方案能快速获得可靠结果。
可选预混液中含有或不含ROX染料的试剂盒(见下表)。
| ROX染料 | 试剂盒 | 兼容的PCR仪 |
|---|---|---|
| 预混液中含有ROX染料 | QuantiTect Virus Kit | 除Applied Biosystems 7500以外的Applied Biosystems的各种PCR仪 |
| 预混液中不含ROX染料 | QuantiTect Virus +ROX Vial Kit | Applied Biosystems 7500和 Bio-Rad、Cepheid、 Eppendorf、QIAGEN, Roche、Agilent等PCR仪 |
我们推荐使用QIAGEN OneStep RT-PCR Kit进行快速、高灵敏度的终点式一步法RT-PCR,包括病毒检测。
应用
QuantiTect Virus Kit可进行高度灵敏的单一或多重real-time PCR,或应用序列特异性探针的一步法RT-PCR,检测内参和病毒DNA和/或RNA。该试剂盒可用于各种real-time PCR仪,包括Applied Biosystems、Bio-Rad、Cepheid、Eppendorf、QIAGEN、Roche(除capillary cyclers以外)和Agilent的PCR仪。
辅助数据和图表
Unambiguous determination of CT values over a wide dynamic range.
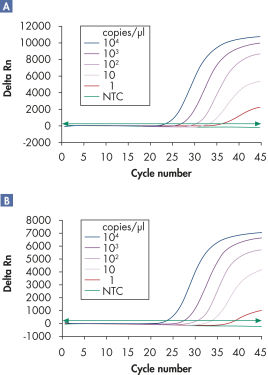
Specifications
| Features | Specifications |
|---|---|
| ApplicationsZH | Virus detection |
| SYBR Green I or sequence-specific probes | Sequence-specific probes |
| Real-time or endpoint | Real-Time |
| Reaction type | Reverse Transcription and PCR |
| Thermal cycler | Most real-time cyclers (except capillary cyclers e.g. LightCycler® 1.x and 2.0) |
| Sample/target type | RNA and/or DNA targets |
| With or without ROX | Available with ROX in master mix and ROX as a separate vial |
| Single or multiplex | Single or multiplex |