✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
AdnaTest ProstateCancerPanel AR-V7
Cat. No. / ID: 396132
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Features
- Efficient isolation and detection of CTCs from whole blood
- Molecular characterization of CTCs
- High specificity and sensitivity
Product Details
Performance
Principle
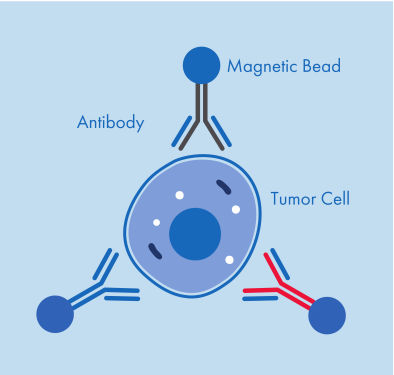
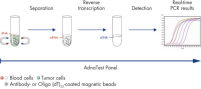
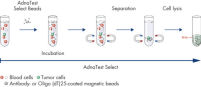
AdnaTest ProstateCancerSelect enables the immunomagnetic enrichment of tumor cells via epithelial and tumor associated antigens. Antibodies against these antigens are conjugated to magnetic beads for labeling of tumor cells in whole blood. Labeled cells are extracted by a magnetic particle concentrator (AdnaMag-L and AdnaMag-S) and are subsequently lysed (“AdnaTest ProstateCancerSelect: Immunomagnetic cell selection with multiple tumor associated antibodies.”). The cell lysate is used for further analysis with AdnaTest ProstateCancerPanel AR-V7.
AdnaTest ProstateCancerPanel AR-V7 contains Oligo (dT)25 Beads for the isolation of mRNA from the lysate of pre-enriched tumor cells. Reverse transcription results in cDNA, which is subsequently used as template for preamplification and tumor cell detection and characterization by qRT-PCR. The AdnaPanel PrimerMixes allow amplification of four tumor associated antigens and two control genes. Furthermore, the kit contains PrimerMixes for amplification of an internal control and an inhibition control. See "AdnaTest ProstateCancerPanel AR-V7: qRT-PCR of AR-V7 and various prostate cancer associated tumor markers."
Procedure
Recovery
The AdnaTest ProstateCancerSelect is used for the enrichment of CTCs from whole blood in prostate cancer research. Subsequently, the AdnaTest ProstateCancerPanel AR-V7 is used for molecular characterization by analyzing gene expression of AR-V7 and further prostate cancer associated genes. In spiking experiments, 2 tumor cells in 5 ml of whole blood are detected at a recovery rate of 95%. >
| Recovery [%] | n | |
|---|---|---|
| 2 tumor cells spiked into 5 ml blood | 38/40 (95%) | 40 |
Two cancer cells (Prostate cancer cell line LnCap) were spiked into blood samples (5 ml) from healthy donors (n=40) to determine the recovery rates achieved with the AdnaTest ProstateCancerPanel AR-V7 as summarized in above. >
Specificity
Fourteen male healthy donors were analyzed with the AdnaTest ProstateCancerPanel AR-V7 to determine the rate of false positives/specificity at the given cut offs. >
| PSA | PSMA | ARwt | AR-V7 | |
|---|---|---|---|---|
| Cut-off | 35.00 | 35.00 | 35.00 | 35.00 |
| Positives after cut-off | 0 | 0 | 1 | 0 |
| Specificity | 100% | 100% | 93% | 100% |
The AdnaTest ProstateCancerPanel AR-V7 could demonstrate in this performance evaluation a specificity of 100% for all tumor associated genes (PSA, PSMA, AR-V7) except ARwt where it reached 93% (details in table above). Also, GAPDH reached 100% specificity and CD45 79%, respectively, whereas these 2 genes are not used for CTC characterization as such, hence a specificity below 90% for those two target genes is acceptable. >
qRT-PCR linearity and efficiency
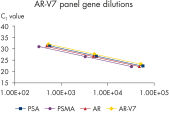
Perfect linearity and efficiency of the qRT-PCR reactions for each of the genes contained in the AdnaTest ProstateCancerPanel AR-V7 was proven in a serial dilution series of standards (See “AR-V7 Panel gene dilutions”). The positive control has been used as template for standard sample generation.
The resulting Ct values for this dilution series were clearly linear, correlated to the copy numbers for each gene at each dilution step (See “AR-V7 Panel gene dilutions”) getting down to ~500 copies. The qRT-PCR efficiency of all the tumor cell related genes was higher than 90% and the corresponding amplification factors ranged above 1.9 which all in all can be taken as a prove for very effective and sensitive qRT-PCR design (see the table below).>
| PSA | PSMA | AR | AR-V7 | |
|---|---|---|---|---|
| Slope over the range | -3.55 | -3.58 | -3.56 | -3.48 |
| Efficiency: -1+10(-1/slope) | 91% | 90% | 91% | 94% |
| Amplification factor: 10^(1/-slope) | 1.91 | 1.90 | 1.91 | 1.94 |
Field test results
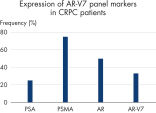
The AdnaTest ProstateCancerPanel AR-V7 workflow was tested in 12 castration-resistant prostate cancer (CRPC) samples. See "Gene profile in clinical samples.">
Applications
- Isolation of CTCs from whole human blood
- Molecular characterization of CTCs
- Biomarker discovery
- mRNA profiling
- Liquid biopsy
- Cancer research
Supporting data and figures
Figure 1: A CTC captured by three antibodies coupled to magnetic beads.