QIAstat-Dx – effortlessly powering results
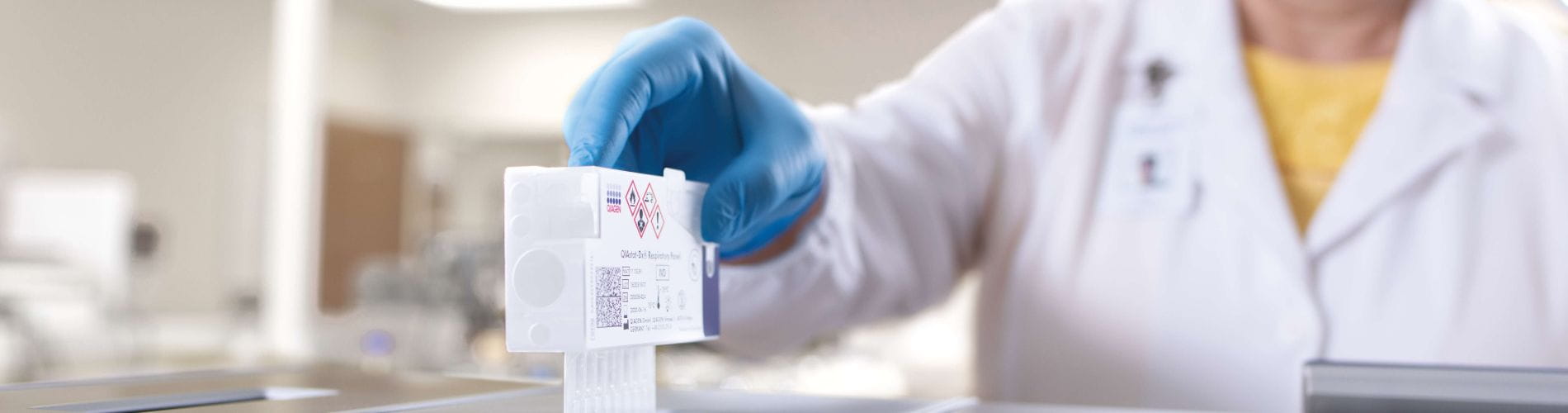
Everything you need in one easy-to-use cartridge
Set your clinical lab up for success with a streamlined syndromic test. The QIAstat-Dx Panels redefine ease-of-use with less than one minute hands-on time and a seamless sample to result workflow. Our innovative cartridge design includes all the reagents on board – ready to use. Your lab will easily adapt to molecular testing demands with minimal training and no precision pipetting required.
With QIAstat-Dx panels, you can:
Maximize your impact on patient care with syndromic testing
With multiplex PCR testing, your lab can accelerate results and gain deeper insights compared to traditional methods. Empower your medical team with results delivered in time to be of clinical value in decision making.Learn more about the benefits of syndromic testing
Reimagine your laboratory workflows with syndromic solutions.
In their words – what our customers have to say about using the QIAstat-Dx

“It became a very easy decision once we learned how sensitive and specific the QIAstat-Dx is and how easy it is to use.”
Michelle Volk, CEO and President of Great Lakes Labs, USA

“The device is exactly what we need. It is so easy to use that after a short training, laboratory professionals in the medical team can easily operate it.”
Luca Sabatini, Medical Services Operator, Carnival Maritime, Italy
Want to see more?
Get a closer look at the QIAstat-Dx Analyzer and QIAstat-Dx panels with our interactive demo.
Additional QIAstat-Dx resources
For up-to-date licensing information and product-specific disclaimers, see the respective QIAGEN kit instructions for use or user manual. QIAGEN instructions for use and user manuals are available at www.qiagen.com or can be requested from QIAGEN Technical Services (or your local distributor).

