Ni-NTA Fast Start Kit
Pour la purification et la détection des protéines recombinantes marquées à l’histidine (His) à partir de lysats d’E. coli
Pour la purification et la détection des protéines recombinantes marquées à l’histidine (His) à partir de lysats d’E. coli
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
Cat. No. / ID: 30600
✓ 24/7 automatic processing of online orders
✓ Knowledgeable and professional Product & Technical Support
✓ Fast and reliable (re)-ordering
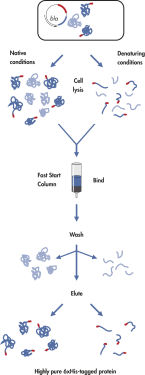
Le Ni-NTA Fast Start Kit est basé sur la remarquable sélectivité de la résine brevetée Ni-NTA (Nickel-Acide nitrilotriacétique) pour les protéines contenant une étiquette d’affinité de six résidus d’histidine (consécutifs ou alternatifs) ou plus, le His-tag. Cette technologie permet une purification en une étape de quasiment toutes les protéines marquées à l’histidine en conditions natives ou dénaturantes. Le NTA, qui présente quatre sites de chélation pour les ions nickel, lie le nickel plus étroitement que les systèmes de purification par chélation du métal, qui ne présentent que trois sites pour l’interaction avec les ions métalliques. Le site de chélation supplémentaire empêche la lixiviation des ions nickel et offre des préparations des protéines d’une pureté supérieure à celle obtenue avec d’autres systèmes de purification par chélation du métal. Le His-tag forme aussi l’épitope reconnu par l’anticorps anti-pentahistidine présent dans le kit.
Le Ni-NTA Fast Start Kit permet une purification fiable en une étape des protéines marquées à l’hexahistidine (6xHis), adaptée à de nombreuses applications, notamment :
| Features | Specifications |
|---|---|
| Applications | Protéomique |
| Support/matrix | Matrice Ni-NTA |
| Processing | Manuel |
| Binding capacity | 5 à 20 mg/ml |
| Special feature | Technologie Ni-NTA |
| Gravity flow or spin column | Gravité |
| Start material | Lysat cellulaire |
| Tag | 6xHis-tag |
| Yield | < 10 mg de protéine par colonne |