QIAGEN Multiplex PCR Kit
For highly specific and sensitive multiplex PCR without optimization requirements
For highly specific and sensitive multiplex PCR without optimization requirements
Cat. No. / ID: 206143
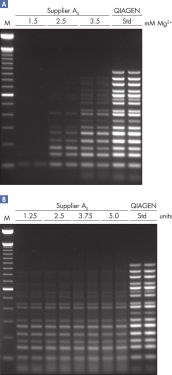
The QIAGEN Multiplex PCR Kit outperformed kits tested from other suppliers and ensures highly specific and sensitive multiplex PCR amplification (see figure " Successful 16-plex PCR "). The kit can be successfully used for various multiplex applications such as typing of transgenic organisms (see figure " Genotyping transgenic mice") and microsatellite analysis (see figure figure " Successful microsatellite analysis "). The master mix includes HotStarTaq DNA Polymerase for efficient amplification of multiple targets in parallel. Amplfication effiency is further improved by an innovative PCR buffer, also included in the master mix. The unique buffer ensures PCR specificity over a wide range of PCR conditions, minimizing the need for optimization. Suboptimal PCR can be improved with Q-Solution — an additive for the amplification of GC-rich templates — also provided with the kit .
Concentration: 5 units/µl
Recombinant enzyme: Yes
Substrate analogs: dNTP, ddNTP, dUTP, biotin-11-dUTP, DIG-11-dUTP, fluorescent-dNTP/ddNTP
Extension rate: 2–4 kb/min at 72°C
Half-life: 10 min at 97°C ; 60 min at 94°C
Amplification efficiency: ≥105 fold
5'–>3' exonuclease activity: Yes
Extra A addition: Yes
3'–>5' exonuclease activity: No
Contaminating nucleases: No
Contaminating RNases: No
Contaminating proteases: No
Self-priming activity: No >
The QIAGEN Multiplex PCR Kit is the first kit specifically developed for multiplex PCR and is provided in an easy-to-use master-mix format. QIAGEN Multiplex PCR Master Mix contains preoptimized concentrations of HotStarTaq DNA Polymerase and MgCl2, plus dNTPs and an innovative PCR buffer specially developed for multiplex PCR. The kit enables success in multiplex PCR at the first attempt. There is no need to optimize reaction conditions (e.g., the concentrations of primers, Mg2+, and Taq DNA polymerase) and cycling parameters due to unique preoptimized reagents included in the kit.
HotStarTaq DNA Polymerase is a modified form of Taq DNA polymerase and has no polymerase activity at ambient temperatures. This prevents extension of nonspecifically annealed primers and primer dimers formed at low temperatures during PCR setup and the initial PCR cycle. HotStarTaq DNA Polymerase is activated by a 15-minute incubation at 95°C which can be incorporated into any existing thermal-cycler program.
This special buffer contains an optimized combination of K+ and NH4+, as well as the unique PCR additive, Factor MP, which increases the local concentration of primers at the template. Together with K+ and other cations, Factor MP stabilizes specifically bound primers, allowing efficient primer extension by HotStarTaq DNA Polymerase (see figure " Stable and efficient primer annealing"). The innovative buffer maintains specific amplification in every cycle of PCR by promoting a high ratio of specific-to-nonspecific primer binding during the annealing step in each PCR cycle. Owing to a uniquely balanced combination of KCl and (NH4)2SO4, the buffer provides stringent primer-annealing conditions over a wider range of annealing temperatures and Mg2+ concentrations than conventional PCR buffers. Optimization of PCR by varying the annealing temperature or the Mg2+ concentration is therefore often minimal or not required.
Q-Solution, an innovative PCR additive that facilitates amplification of difficult templates by modifying the melting behavior of DNA, is also provided with HotStarTaq DNA Polymerase. This unique reagent improves suboptimal PCR caused by templates that have a high degree of secondary structure or GC-rich templates. Unlike other commonly used PCR additives such as DMSO, Q-Solution is used at just one working concentration, is nontoxic, and PCR purity is guaranteed.
The QIAGEN Multiplex PCR Kit is provided in a ready-to-use, preoptimized master mix for greater convenience. Use of a master mix saves time, simplifies handling for reaction setup, and increases reproducibility by eliminating many possible sources of pipetting errors and contamination — pipetting steps are minimized and tedious calculations are eliminated. Only primers and template need to be added to prepare the final amplification mix. The master mix can be stored at
2–8°C, allowing even faster setup of multiplex PCR assays. The streamlined, step-by-step protocol provided with the kit ensures fast and easy PCR setup. Reactions can be set up at room temperature, ensuring greater convenience and ease of use. HotStarTaq DNA Polymerase is activated by a 15-minute, 95°C incubation step, which can easily be incorporated into existing thermal cycling programs.
The QIAGEN Multiplex PCR Kit is highly suited for various multiplex applications, including:
| Features | Specifications |
|---|---|
| Applications | PCR, RT-PCR, multiplex PCR, typing, detection |
| Enzyme activity | 5' -> 3' exonuclease activity |
| Reaction type | PCR amplification |
| With/without hotstart | With hotstart |
| Single or multiplex | Multiplex |
| Real-time or endpoint | Endpoint |
| Mastermix | Yes |
| Sample/target type | Genomic DNA and cDNA |